Analysis of spatiotemporal pattern and quantification of gastrointestinal slow waves caused by anticholinergic drugs
- PMID: 28277890
- PMCID: PMC5465949
- DOI: 10.1080/15476278.2017.1295904
Analysis of spatiotemporal pattern and quantification of gastrointestinal slow waves caused by anticholinergic drugs
Abstract
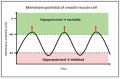
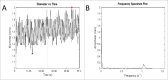
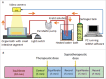
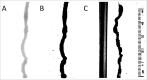
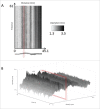
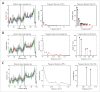
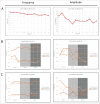
Anticholinergic drugs are well-known to cause adverse effects, such as constipation, but their effects on baseline contractile activity in the gut driven by slow waves is not well established. In a video-based gastrointestinal motility monitoring (GIMM) system, a mouse's small intestine was placed in Krebs solution and recorded using a high definition camera. Untreated controls were recorded for each specimen, then treated with a therapeutic concentration of the drug, and finally, treated with a supratherapeutic dose of the drug. Next, the video clips showing gastrointestinal motility were processed, giving us the segmentation motions of the intestine, which were then converted via Fast Fourier Transform (FFT) into their respective frequency spectrums. These contraction quantifications were analyzed from the video recordings under standardised conditions to evaluate the effect of drugs. Six experimental trials were included with benztropine and promethazine treatments. Only the supratherapeutic dose of benztropine was shown to significantly decrease the amplitude of contractions; at therapeutic doses of both drugs, neither frequency nor amplitude was significantly affected. We have demonstrated that intestinal slow waves can be analyzed based on the colonic frequency or amplitude at a supratherapeutic dose of the anticholinergic medications. More research is required on the effects of anticholinergic drugs on these slow waves to ascertain the true role of ICC in neurologic control of gastrointestinal motility.
Keywords: anticholinergic drugs; frequency domain; gastrointestinal motility monitoring system; interstitial cells of Cajal; spatiotemporal analysis.
Figures



Similar articles
-
High-Dose Radiation-Induced Changes in Murine Small Intestinal Motility: Are the Changes in the Interstitial Cells of Cajal or in the Enteric Nervous System?Radiat Res. 2016 Jan;185(1):39-49. doi: 10.1667/RR14132.1. Epub 2015 Dec 31. Radiat Res. 2016. PMID: 26720798
-
Computer vision-based diameter maps to study fluoroscopic recordings of small intestinal motility from conscious experimental animals.Neurogastroenterol Motil. 2017 Aug;29(8). doi: 10.1111/nmo.13052. Epub 2017 Mar 16. Neurogastroenterol Motil. 2017. PMID: 28300332
-
Herbal extracts modulate the amplitude and frequency of slow waves in circular smooth muscle of mouse small intestine.Digestion. 2004;70(4):257-64. doi: 10.1159/000083718. Epub 2005 Jan 8. Digestion. 2004. PMID: 15687728
-
Are interstitial cells of Cajal plurifunction cells in the gut?Am J Physiol Gastrointest Liver Physiol. 2008 Feb;294(2):G372-90. doi: 10.1152/ajpgi.00344.2007. Epub 2007 Oct 11. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 17932226 Review.
-
[Gastrointestinal motility disorders and drugs: cholinergic, anticholinergic, adrenergic, antiadrenergic and prokinetic drugs (part I)].Srp Arh Celok Lek. 1994 Jan-Feb;122(1-2):50-4. Srp Arh Celok Lek. 1994. PMID: 17972806 Review. Serbian.
Cited by
-
Influence of Acetylcholine Esterase Inhibitors and Memantine, Clinically Approved for Alzheimer's Dementia Treatment, on Intestinal Properties of the Mouse.Int J Mol Sci. 2021 Jan 20;22(3):1015. doi: 10.3390/ijms22031015. Int J Mol Sci. 2021. PMID: 33498392 Free PMC article.
-
Trihexyphenidyl Alters Its Host's Metabolism, Neurobehavioral Patterns, and Gut Microbiome Feedback Loop-The Modulating Role of Anacyclus pyrethrum.Antioxidants (Basel). 2023 Dec 21;13(1):26. doi: 10.3390/antiox13010026. Antioxidants (Basel). 2023. PMID: 38275646 Free PMC article.
-
A deep learning based framework for the classification of multi- class capsule gastroscope image in gastroenterologic diagnosis.Front Physiol. 2022 Nov 18;13:1060591. doi: 10.3389/fphys.2022.1060591. eCollection 2022. Front Physiol. 2022. PMID: 36467700 Free PMC article.
References
-
- Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol 2010; 298(1):G10-G3; http://dx.doi.org/10.1152/ajpgi.00426.2009 - DOI - PMC - PubMed
-
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterol 1996; 111(2):492-515; http://dx.doi.org/10.1053/gast.1996.v111.pm8690216 - DOI - PubMed
-
- Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 2010; 588(23):4621-39; PMID:20921202; http://dx.doi.org/10.1113/jphysiol.2010.196030 - DOI - PMC - PubMed
-
- Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol 2008; 294(2):G372-G90; PMID:17932226; http://dx.doi.org/10.1152/ajpgi.00344.2007 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
