In Vivo Bioluminescence Imaging for Longitudinal Monitoring of Inflammation in Animal Models of Uveitis
- PMID: 28278321
- PMCID: PMC5361579
- DOI: 10.1167/iovs.16-20824
In Vivo Bioluminescence Imaging for Longitudinal Monitoring of Inflammation in Animal Models of Uveitis
Abstract
Purpose: We develop a quantitative bioluminescence assay for in vivo longitudinal monitoring of inflammation in animal models of uveitis.
Methods: Three models of experimental uveitis were induced in C57BL/6 albino mice: primed mycobacterial uveitis (PMU), endotoxin-induced uveitis (EIU), and experimental autoimmune uveitis (EAU). Intraperitoneal injection of luminol sodium salt, which emits light when oxidized, provided the bioluminescence substrate. Bioluminescence images were captured by a PerkinElmer In Vivo Imaging System (IVIS) Spectrum and total bioluminescence was analyzed using Living Image software. Bioluminescence on day zero was compared to bioluminescence on the day of peak inflammation for each model. Longitudinal bioluminescence imaging was performed in EIU and EAU.
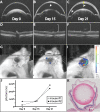
Results: In the presence of luminol, intraocular inflammation generates detectable bioluminescence in three mouse models of uveitis. Peak bioluminescence in inflamed PMU eyes (1.46 × 105 photons/second [p/s]) was significantly increased over baseline (1.47 × 104 p/s, P = 0.01). Peak bioluminescence in inflamed EIU eyes (3.18 × 104 p/s) also was significantly increased over baseline (1.09 × 104 p/s, P = 0.04), and returned to near baseline levels by 48 hours. In EAU, there was a nonsignificant increase in bioluminescence at peak inflammation.
Conclusions: In vivo bioluminescence may be used as a noninvasive, quantitative measure of intraocular inflammation in animal models of uveitis. Primed mycobacterial uveitis and EIU are both acute models with robust anterior inflammation and demonstrated significant changes in bioluminescence corresponding with peak inflammation. Experimental autoimmune uveitis is a more indolent posterior uveitis and generated a more modest bioluminescent signal. In vivo imaging system bioluminescence is a nonlethal, quantifiable assay that can be used for monitoring inflammation in animal models of uveitis.
Figures



References
-
- Dick AD,, Cheng YF,, McKinnon A,, Liversidge J,, Forrester JV. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Ophthalmol. 1993; 77: 171–175. - PMC - PubMed
-
- Xu H,, Koch P,, Chen M,, Lau A,, Reid DM,, Forrester JV. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res. 2008; 87: 319–326. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

