Potential Metabolic Activation of a Representative C4-Alkylated Polycyclic Aromatic Hydrocarbon Retene (1-Methyl-7-isopropyl-phenanthrene) Associated with the Deepwater Horizon Oil Spill in Human Hepatoma (HepG2) Cells
- PMID: 28278373
- PMCID: PMC5593134
- DOI: 10.1021/acs.chemrestox.6b00457
Potential Metabolic Activation of a Representative C4-Alkylated Polycyclic Aromatic Hydrocarbon Retene (1-Methyl-7-isopropyl-phenanthrene) Associated with the Deepwater Horizon Oil Spill in Human Hepatoma (HepG2) Cells
Abstract
Exposure to petrogenic polycyclic aromatic hydrocarbons (PPAHs) in the food chain is the major human health hazard associated with the Deepwater Horizon oil spill. C4-Phenanthrenes are representative PPAHs present in the crude oil and could contaminate the seafood. We describe the metabolism of a C4-phenanthrene regioisomer retene (1-methyl-7-isopropyl-phenanthrene) in human HepG2 cells as a model for metabolism in human hepatocytes. Retene because of its sites of alkylation cannot be metabolized to a diol-epoxide. The structures of the metabolites were identified by HPLC-UV-fluorescence detection and LC-MS/MS. O-Monosulfonated-retene-catechols were discovered as signature metabolites of the ortho-quinone pathway of PAH activation catalyzed by aldo-keto reductases. We also found evidence for the formation of bis-ortho-quinones where the two dicarbonyl groups were present on different rings of retene. The identification of O-monosulfonated-retene-catechol and O-bismethyl-O-monoglucuronosyl-retene-bis-catechol supports metabolic activation of retene by P450 and aldo-keto reductase isozymes followed by metabolic detoxification of the ortho-quinone through interception of redox cycling by catechol-O-methyltransferase, uridine 5'-diphospho-glucuronosyltransferase, and sulfotransferase isozymes. We propose that catechol conjugates could be used as biomarkers of human exposure to retene resulting from oil spills.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





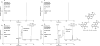


References
-
- Joye SB, MacDonald IR, Leifer I, Asper V. Magnitude and oxidation potential of hydrocarbon gases released from the BP oil well blowout. Nat Geosci. 2011;4:160–164.
-
- Reddy CM, Arey JS, Seewald JS, Sylva SP, Lemkau KL, Nelson RK, Carmichael CA, McIntyre CP, Fenwick J, Ventura GT, Van Mooy BA, Camilli R. Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proc Natl Acad Sci U S A. 2012;109:20229–20234. - PMC - PubMed
-
- Ryerson TB, Camilli R, Kessler JD, Kujawinski EB, Reddy CM, Valentine DL, Atlas E, Blake DR, de Gouw J, Meinardi S, Parrish DD, Peischl J, Seewald JS, Warneke C. Chemical data quantify Deepwater Horizon hydrocarbon flow rate and environmental distribution. Proc Natl Acad Sci U S A. 2012;109:20246–20253. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

