Engineered microenvironments for synergistic VEGF - Integrin signalling during vascularization
- PMID: 28279265
- PMCID: PMC5354119
- DOI: 10.1016/j.biomaterials.2017.02.024
Engineered microenvironments for synergistic VEGF - Integrin signalling during vascularization
Abstract
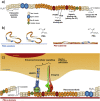
We have engineered polymer-based microenvironments that promote vasculogenesis both in vitro and in vivo through synergistic integrin-growth factor receptor signalling. Poly(ethyl acrylate) (PEA) triggers spontaneous organization of fibronectin (FN) into nanonetworks which provide availability of critical binding domains. Importantly, the growth factor binding (FNIII12-14) and integrin binding (FNIII9-10) regions are simultaneously available on FN fibrils assembled on PEA. This material platform promotes synergistic integrin/VEGF signalling which is highly effective for vascularization events in vitro with low concentrations of VEGF. VEGF specifically binds to FN fibrils on PEA compared to control polymers (poly(methyl acrylate), PMA) where FN remains in a globular conformation and integrin/GF binding domains are not simultaneously available. The vasculogenic response of human endothelial cells seeded on these synergistic interfaces (VEGF bound to FN assembled on PEA) was significantly improved compared to soluble administration of VEGF at higher doses. Early onset of VEGF signalling (PLCγ1 phosphorylation) and both integrin and VEGF signalling (ERK1/2 phosphorylation) were increased only when VEGF was bound to FN nanonetworks on PEA, while soluble VEGF did not influence early signalling. Experiments with mutant FN molecules with impaired integrin binding site (FN-RGE) confirmed the role of the integrin binding site of FN on the vasculogenic response via combined integrin/VEGF signalling. In vivo experiments using 3D scaffolds coated with FN and VEGF implanted in the murine fat pad demonstrated pro-vascularization signalling by enhanced formation of new tissue inside scaffold pores. PEA-driven organization of FN promotes efficient presentation of VEGF to promote vascularization in regenerative medicine applications.
Keywords: Fibronectin; Growth factors; Protein assembly; VEGF; Vascularization; poly(ethyl acrylate).
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures








Similar articles
-
Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis.Development. 2011 Oct;138(20):4451-63. doi: 10.1242/dev.071381. Epub 2011 Aug 31. Development. 2011. PMID: 21880786 Free PMC article.
-
Glycation of fibronectin inhibits VEGF-induced angiogenesis by uncoupling VEGF receptor-2-c-Src crosstalk.J Cell Mol Med. 2020 Aug;24(16):9154-9164. doi: 10.1111/jcmm.15552. Epub 2020 Jul 1. J Cell Mol Med. 2020. PMID: 32613750 Free PMC article.
-
A material-based platform to modulate fibronectin activity and focal adhesion assembly.Biores Open Access. 2014 Dec 1;3(6):286-96. doi: 10.1089/biores.2014.0033. Biores Open Access. 2014. PMID: 25469314 Free PMC article.
-
The ins and outs of fibronectin matrix assembly.J Cell Sci. 2003 Aug 15;116(Pt 16):3269-76. doi: 10.1242/jcs.00670. J Cell Sci. 2003. PMID: 12857786 Review.
-
The role of integrin binding sites in fibronectin matrix assembly in vivo.Curr Opin Cell Biol. 2008 Oct;20(5):502-7. doi: 10.1016/j.ceb.2008.06.001. Epub 2008 Jul 21. Curr Opin Cell Biol. 2008. PMID: 18586094 Review.
Cited by
-
Surface-Modified Piezoelectric Copolymer Poly(vinylidene fluoride-trifluoroethylene) Supporting Physiological Extracellular Matrixes to Enhance Mesenchymal Stem Cell Adhesion for Nanoscale Mechanical Stimulation.ACS Appl Mater Interfaces. 2023 Nov 8;15(44):50652-50662. doi: 10.1021/acsami.3c05128. Epub 2023 Sep 17. ACS Appl Mater Interfaces. 2023. PMID: 37718477 Free PMC article. Review.
-
Cell-Laden Gradient Hydrogel Scaffolds for Neovascularization of Engineered Tissues.Adv Healthc Mater. 2021 Apr;10(7):e2001706. doi: 10.1002/adhm.202001706. Epub 2021 Jan 29. Adv Healthc Mater. 2021. PMID: 33511790 Free PMC article.
-
Biologically Inspired, Cell-Selective Release of Aptamer-Trapped Growth Factors by Traction Forces.Adv Mater. 2019 Feb;31(7):e1806380. doi: 10.1002/adma.201806380. Epub 2019 Jan 7. Adv Mater. 2019. PMID: 30614086 Free PMC article.
-
Nanoscale Coatings for Ultralow Dose BMP-2-Driven Regeneration of Critical-Sized Bone Defects.Adv Sci (Weinh). 2018 Nov 19;6(2):1800361. doi: 10.1002/advs.201800361. eCollection 2019 Jan 23. Adv Sci (Weinh). 2018. PMID: 30693176 Free PMC article.
-
Mechanisms of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (huang qi) and Angelica sinensis (Oliv.) Diels (dang gui) in Ameliorating Hypoxia and Angiogenesis to Delay Pulmonary Nodule Malignant Transformation.Integr Cancer Ther. 2025 Jan-Dec;24:15347354241311917. doi: 10.1177/15347354241311917. Integr Cancer Ther. 2025. PMID: 39882753 Free PMC article. Review.
References
-
- Auger F.A., Gibot L., Lacroix D. The pivotal role of vascularization in tissue engineering. Annu. Rev. Biomed. Eng. 2013;15:177–200. - PubMed
-
- Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7:359–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

