Structure-Guided Identification of a Family of Dual Receptor-Binding PfEMP1 that Is Associated with Cerebral Malaria
- PMID: 28279348
- PMCID: PMC5374107
- DOI: 10.1016/j.chom.2017.02.009
Structure-Guided Identification of a Family of Dual Receptor-Binding PfEMP1 that Is Associated with Cerebral Malaria
Abstract
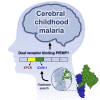
Cerebral malaria is a deadly outcome of infection by Plasmodium falciparum, occurring when parasite-infected erythrocytes accumulate in the brain. These erythrocytes display parasite proteins of the PfEMP1 family that bind various endothelial receptors. Despite the importance of cerebral malaria, a binding phenotype linked to its symptoms has not been identified. Here, we used structural biology to determine how a group of PfEMP1 proteins interacts with intercellular adhesion molecule 1 (ICAM-1), allowing us to predict binders from a specific sequence motif alone. Analysis of multiple Plasmodium falciparum genomes showed that ICAM-1-binding PfEMP1s also interact with endothelial protein C receptor (EPCR), allowing infected erythrocytes to synergistically bind both receptors. Expression of these PfEMP1s, predicted to bind both ICAM-1 and EPCR, is associated with increased risk of developing cerebral malaria. This study therefore reveals an important PfEMP1-binding phenotype that could be targeted as part of a strategy to prevent cerebral malaria.
Keywords: EPCR; ICAM-1; PfEMP1; Plasmodium falciparum; cerebral malaria.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Avril M., Tripathi A.K., Brazier A.J., Andisi C., Janes J.H., Soma V.L., Sullivan D.J., Jr., Bull P.C., Stins M.F., Smith J.D. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc. Natl. Acad. Sci. USA. 2012;109:E1782–E1790. - PMC - PubMed
-
- Bengtsson A., Joergensen L., Rask T.S., Olsen R.W., Andersen M.A., Turner L., Theander T.G., Hviid L., Higgins M.K., Craig A. A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J. Immunol. 2013;190:240–249. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

