A small-molecule IRF3 agonist functions as an influenza vaccine adjuvant by modulating the antiviral immune response
- PMID: 28279563
- PMCID: PMC11514956
- DOI: 10.1016/j.vaccine.2017.01.053
A small-molecule IRF3 agonist functions as an influenza vaccine adjuvant by modulating the antiviral immune response
Abstract
Vaccine adjuvants are essential to drive a protective immune response in cases where vaccine antigens are weakly immunogenic, where vaccine antigen is limited, or where an increase in potency is needed for a specific population, such as the elderly. To discover novel vaccine adjuvants, we used a high-throughput screen (HTS) designed to identify small-molecule agonists of the RIG-I-like receptor (RLR) pathway leading to interferon regulatory factor 3 (IRF3) activation. RLRs are a group of cytosolic pattern-recognition receptors that are essential for the recognition of viral nucleic acids during infection. Upon binding of viral nucleic acid ligands, the RLRs become activated and signal to transcription factors, including IRF3, to initiate an innate immune transcriptional program to control virus infection. Among our HTS hits were a series of benzothiazole compounds from which we designed the lead analog, KIN1148. KIN1148 induced dose-dependent IRF3 nuclear translocation and specific activation of IRF3-responsive promoters. Prime-boost immunization of mice with a suboptimal dose of a monovalent pandemic influenza split virus H1N1 A/California/07/2009 vaccine plus KIN1148 protected against a lethal challenge with mouse-adapted influenza virus (A/California/04/2009) and induced an influenza virus-specific IL-10 and Th2 response by T cells derived from lung and lung-draining lymph nodes. Prime-boost immunization with vaccine plus KIN1148, but not prime immunization alone, induced antibodies capable of inhibiting influenza virus hemagglutinin and neutralizing viral infectivity. Nevertheless, a single immunization with vaccine plus KIN1148 provided increased protection over vaccine alone and reduced viral load in the lungs after challenge. These findings suggest that protection was at least partially mediated by a cellular immune component and that the induction of Th2 and immunoregulatory cytokines by a KIN1148-adjuvanted vaccine may be particularly beneficial for ameliorating the immunopathogenesis that is associated with influenza viruses.
Keywords: Adjuvant; IRF3; Influenza; Vaccine.
Copyright © 2017. Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest
PP, JG, MW, MG, SI, and KB own equity shares in Kineta, Inc.
Figures
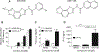
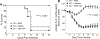
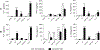



References
-
- World Health Organization. Influenza fact sheet; 2016. http://www.who.int/mediacentre/factsheets/fs211/en/ [accessed December 21, 2016].
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

