Follistatin Gene Therapy for Sporadic Inclusion Body Myositis Improves Functional Outcomes
- PMID: 28279643
- PMCID: PMC5383643
- DOI: 10.1016/j.ymthe.2017.02.015
Follistatin Gene Therapy for Sporadic Inclusion Body Myositis Improves Functional Outcomes
Abstract
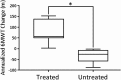
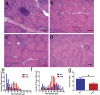
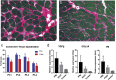
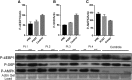
Sporadic inclusion body myositis, a variant of inflammatory myopathy, has features distinct from polymyositis/dermatomyositis. The disease affects men more than women, most commonly after age 50. Clinical features include weakness of the quadriceps, finger flexors, ankle dorsiflexors, and dysphagia. The distribution of weakness is similar to Becker muscular dystrophy, where we previously reported improvement following intramuscular injection of an isoform of follistatin (FS344) by AAV1. For this clinical trial, rAAV1.CMV.huFS344, 6 × 1011 vg/kg, was delivered to the quadriceps muscles of both legs of six sporadic inclusion body myositis subjects. The primary outcome for this trial was distance traveled for the 6-min walk test. The protocol included an exercise regimen for each participant. Performance, annualized to a median 1-year change, improved +56.0 m/year for treated subjects compared to a decline of -25.8 m/year (p = 0.01) in untreated subjects (n = 8), matched for age, gender, and baseline measures. Four of the six treated subjects showed increases ranging from 58-153 m, whereas two were minimally improved (5-23 m). Treatment effects included decreased fibrosis and improved regeneration. These findings show promise for follistatin gene therapy for mild to moderately affected, ambulatory sporadic inclusion body myositis patients. More advanced disease with discernible muscle loss poses challenges.
Keywords: 6-min walk distance; 6-min walk test; Follistatin; adeno-associated virus; gene therapy; sporadic inclusion body myositis.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Unfounded Claims of Improved Functional Outcomes Attributed to Follistatin Gene Therapy in Inclusion Body Myositis.Mol Ther. 2017 Oct 4;25(10):2235-2237. doi: 10.1016/j.ymthe.2017.09.002. Epub 2017 Sep 8. Mol Ther. 2017. PMID: 28927986 Free PMC article. No abstract available.
-
Reply to Letter to the Editor.Mol Ther. 2017 Oct 4;25(10):2238-2240. doi: 10.1016/j.ymthe.2017.09.003. Epub 2017 Sep 8. Mol Ther. 2017. PMID: 28939086 Free PMC article. No abstract available.
References
-
- Yunis E.J., Samaha F.J. Inclusion body myositis. Lab. Invest. 1971;25:240–248. - PubMed
-
- Dalakas M.C. Inflammatory muscle diseases: a critical review on pathogenesis and therapies. Curr. Opin. Pharmacol. 2010;10:346–352. - PubMed
-
- Wilson F.C., Ytterberg S.R., St Sauver J.L., Reed A.M. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J. Rheumatol. 2008;35:445–447. - PubMed
-
- Chahin N., Engel A.G. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology. 2008;70:418–424. - PubMed
-
- Griggs R.C., Askanas V., DiMauro S., Engel A., Karpati G., Mendell J.R., Rowland L.P. Inclusion body myositis and myopathies. Ann. Neurol. 1995;38:705–713. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

