Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health
- PMID: 28279944
- PMCID: PMC5343002
- DOI: 10.1016/j.redox.2017.02.011
Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health
Abstract
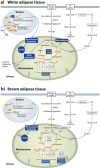
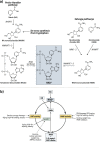
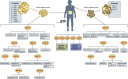
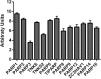
Obesity, a chronic state of energy overload, is characterized by adipose tissue dysfunction that is considered to be the major driver for obesity associated metabolic complications. The reasons for adipose tissue dysfunction are incompletely understood, but one potential contributing factor is adipose tissue mitochondrial dysfunction. Derangements of adipose tissue mitochondrial biogenesis and pathways associate with obesity and metabolic diseases. Mitochondria are central organelles in energy metabolism through their role in energy derivation through catabolic oxidative reactions. The mitochondrial processes are dependent on the proper NAD+/NADH redox balance and NAD+ is essential for reactions catalyzed by the key regulators of mitochondrial metabolism, sirtuins (SIRTs) and poly(ADP-ribose) polymerases (PARPs). Notably, obesity is associated with disturbed adipose tissue NAD+ homeostasis and the balance of SIRT and PARP activities. In this review we aim to summarize existing literature on the maintenance of intracellular NAD+ pools and the function of SIRTs and PARPs in adipose tissue during normal and obese conditions, with the purpose of comprehending their potential role in mitochondrial derangements and obesity associated metabolic complications. Understanding the molecular mechanisms that are the root cause of the adipose tissue mitochondrial derangements is crucial for developing new effective strategies to reverse obesity associated metabolic complications.
Keywords: Adipose tissue; Mitochondria; NAD(+); Obesity; Poly(ADP-ribose) polymerases; Sirtuins.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Koh E.H., Park J.-Y., Park H.-S. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56:2973–2981. - PubMed
-
- Wang C.-H., Wang C.-C., Huang H.-C., Wei Y.-H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013;280:1039–1050. - PubMed
-
- Björntorp P., Bengtsson C., Blohmé G. Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism. 1971;20:927–935. - PubMed
-
- Heinonen S., Saarinen L., Naukkarinen J. Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int. J. Obes. 2014;38:1423–1431. - PubMed
-
- Heilbronn L., Smith S.R., Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2004;28:S12–S21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

