Targeted therapy for a subset of acute myeloid leukemias that lack expression of aldehyde dehydrogenase 1A1
- PMID: 28280079
- PMCID: PMC5451337
- DOI: 10.3324/haematol.2016.159053
Targeted therapy for a subset of acute myeloid leukemias that lack expression of aldehyde dehydrogenase 1A1
Abstract
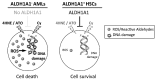
Aldehyde dehydrogenase 1A1 (ALDH1A1) activity is high in hematopoietic stem cells and functions in part to protect stem cells from reactive aldehydes and other toxic compounds. In contrast, we found that approximately 25% of all acute myeloid leukemias expressed low or undetectable levels of ALDH1A1 and that this ALDH1A1- subset of leukemias correlates with good prognosis cytogenetics. ALDH1A1- cell lines as well as primary leukemia cells were found to be sensitive to treatment with compounds that directly and indirectly generate toxic ALDH substrates including 4-hydroxynonenal and the clinically relevant compounds arsenic trioxide and 4-hydroperoxycyclophosphamide. In contrast, normal hematopoietic stem cells were relatively resistant to these compounds. Using a murine xenotransplant model to emulate a clinical treatment strategy, established ALDH1A1- leukemias were also sensitive to in vivo treatment with cyclophosphamide combined with arsenic trioxide. These results demonstrate that targeting ALDH1A1- leukemic cells with toxic ALDH1A1 substrates such as arsenic and cyclophosphamide may be a novel targeted therapeutic strategy for this subset of acute myeloid leukemias.
Copyright© Ferrata Storti Foundation.
Figures





Similar articles
-
Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems.Blood. 2009 Feb 19;113(8):1670-80. doi: 10.1182/blood-2008-05-156752. Epub 2008 Oct 29. Blood. 2009. PMID: 18971422 Free PMC article.
-
The Molecular Context of Oxidant Stress Response in Cancer Establishes ALDH1A1 as a Critical Target: What This Means for Acute Myeloid Leukemia.Int J Mol Sci. 2023 May 27;24(11):9372. doi: 10.3390/ijms24119372. Int J Mol Sci. 2023. PMID: 37298333 Free PMC article. Review.
-
Variable aldehyde dehydrogenase activity and effects on chemosensitivity of primitive human leukemic cells.Exp Hematol. 2017 Mar;47:54-63. doi: 10.1016/j.exphem.2016.10.012. Epub 2016 Nov 5. Exp Hematol. 2017. PMID: 27826122
-
The rarity of ALDH(+) cells is the key to separation of normal versus leukemia stem cells by ALDH activity in AML patients.Int J Cancer. 2015 Aug 1;137(3):525-36. doi: 10.1002/ijc.29410. Epub 2015 Jan 14. Int J Cancer. 2015. PMID: 25545165 Free PMC article.
-
Aldehyde dehydrogenase 1A1 in stem cells and cancer.Oncotarget. 2016 Mar 8;7(10):11018-32. doi: 10.18632/oncotarget.6920. Oncotarget. 2016. PMID: 26783961 Free PMC article. Review.
Cited by
-
Transcriptional Silencing of ALDH2 Confers a Dependency on Fanconi Anemia Proteins in Acute Myeloid Leukemia.Cancer Discov. 2021 Sep;11(9):2300-2315. doi: 10.1158/2159-8290.CD-20-1542. Epub 2021 Apr 23. Cancer Discov. 2021. PMID: 33893150 Free PMC article.
-
Expression of Aldehyde Dehydrogenase 1A1 in Relapse-Associated Cells in Acute Myeloid Leukemia.Cells. 2025 Jul 7;14(13):1038. doi: 10.3390/cells14131038. Cells. 2025. PMID: 40643557 Free PMC article. Review.
-
OGG1 as an Epigenetic Reader Affects NFκB: What This Means for Cancer.Cancers (Basel). 2023 Dec 28;16(1):148. doi: 10.3390/cancers16010148. Cancers (Basel). 2023. PMID: 38201575 Free PMC article. Review.
-
Aldehyde Dehydrogenase Genes as Prospective Actionable Targets in Acute Myeloid Leukemia.Genes (Basel). 2023 Sep 16;14(9):1807. doi: 10.3390/genes14091807. Genes (Basel). 2023. PMID: 37761947 Free PMC article.
-
Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders.Antioxidants (Basel). 2018 Jul 30;7(8):102. doi: 10.3390/antiox7080102. Antioxidants (Basel). 2018. PMID: 30061536 Free PMC article. Review.
References
-
- Gasparetto M, Sekulovic S, Brocker C, et al. Aldehyde dehydrogenases are regulators of hematopoietic stem cell numbers and B-cell development. Exp Hematol. 2012; 40(4):318–329.e312. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

