Topological knots and links in proteins
- PMID: 28280100
- PMCID: PMC5380043
- DOI: 10.1073/pnas.1615862114
Topological knots and links in proteins
Abstract
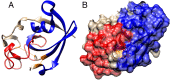
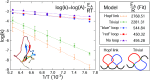
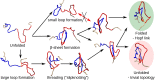
Twenty years after their discovery, knots in proteins are now quite well understood. They are believed to be functionally advantageous and provide extra stability to protein chains. In this work, we go one step further and search for links-entangled structures, more complex than knots, which consist of several components. We derive conditions that proteins need to meet to be able to form links. We search through the entire Protein Data Bank and identify several sequentially nonhomologous chains that form a Hopf link and a Solomon link. We relate topological properties of these proteins to their function and stability and show that the link topology is characteristic of eukaryotes only. We also explain how the presence of links affects the folding pathways of proteins. Finally, we define necessary conditions to form Borromean rings in proteins and show that no structure in the Protein Data Bank forms a link of this type.
Keywords: catenanes; disulphide bridge; folding; lasso; slipknot.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Mansfield ML. Are there knots in proteins? Nat Struc Biol. 1994;1(4):213–214. - PubMed
-
- Mansfield ML. Fit to be tied. Nat Struc Biol. 1997;4(3):166–167. - PubMed
-
- Takusagawa F, Kamitori S. A real knot in protein. J Am Chem Soc. 1996;118(37):8945–8946.
-
- Taylor WR. A deeply knotted protein structure and how it might fold. Nature. 2000;406(6798):916–919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

