Tango1 spatially organizes ER exit sites to control ER export
- PMID: 28280122
- PMCID: PMC5379956
- DOI: 10.1083/jcb.201611088
Tango1 spatially organizes ER exit sites to control ER export
Abstract
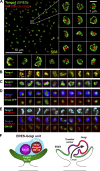
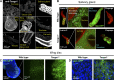
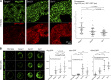
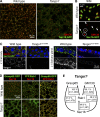
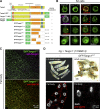
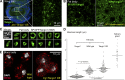
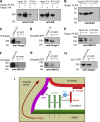
Exit of secretory cargo from the endoplasmic reticulum (ER) takes place at specialized domains called ER exit sites (ERESs). In mammals, loss of TANGO1 and other MIA/cTAGE (melanoma inhibitory activity/cutaneous T cell lymphoma-associated antigen) family proteins prevents ER exit of large cargoes such as collagen. Here, we show that Drosophila melanogaster Tango1, the only MIA/cTAGE family member in fruit flies, is a critical organizer of the ERES-Golgi interface. Tango1 rings hold COPII (coat protein II) carriers and Golgi in close proximity at their center. Loss of Tango1, present at ERESs in all tissues, reduces ERES size and causes ERES-Golgi uncoupling, which impairs secretion of not only collagen, but also all other cargoes we examined. Further supporting an organizing role of Tango1, its overexpression creates more and larger ERESs. Our results suggest that spatial coordination of ERES, carrier, and Golgi elements through Tango1's multiple interactions increases secretory capacity in Drosophila and allows secretion of large cargo.
© 2017 Liu et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

