Cell-Free DNA and Active Rejection in Kidney Allografts
- PMID: 28280140
- PMCID: PMC5491290
- DOI: 10.1681/ASN.2016091034
Cell-Free DNA and Active Rejection in Kidney Allografts
Abstract
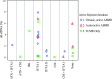
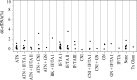
Histologic analysis of the allograft biopsy specimen is the standard method used to differentiate rejection from other injury in kidney transplants. Donor-derived cell-free DNA (dd-cfDNA) is a noninvasive test of allograft injury that may enable more frequent, quantitative, and safer assessment of allograft rejection and injury status. To investigate this possibility, we prospectively collected blood specimens at scheduled intervals and at the time of clinically indicated biopsies. In 102 kidney recipients, we measured plasma levels of dd-cfDNA and correlated the levels with allograft rejection status ascertained by histology in 107 biopsy specimens. The dd-cfDNA level discriminated between biopsy specimens showing any rejection (T cell-mediated rejection or antibody-mediated rejection [ABMR]) and controls (no rejection histologically), P<0.001 (receiver operating characteristic area under the curve [AUC], 0.74; 95% confidence interval [95% CI], 0.61 to 0.86). Positive and negative predictive values for active rejection at a cutoff of 1.0% dd-cfDNA were 61% and 84%, respectively. The AUC for discriminating ABMR from samples without ABMR was 0.87 (95% CI, 0.75 to 0.97). Positive and negative predictive values for ABMR at a cutoff of 1.0% dd-cfDNA were 44% and 96%, respectively. Median dd-cfDNA was 2.9% (ABMR), 1.2% (T cell-mediated types ≥IB), 0.2% (T cell-mediated type IA), and 0.3% in controls (P=0.05 for T cell-mediated rejection types ≥IB versus controls). Thus, dd-cfDNA may be used to assess allograft rejection and injury; dd-cfDNA levels <1% reflect the absence of active rejection (T cell-mediated type ≥IB or ABMR) and levels >1% indicate a probability of active rejection.
Keywords: DART; biomarker; cell-free DNA; kidney; rejection; transplant.
Copyright © 2017 by the American Society of Nephrology.
Figures




Comment in
-
Cell-Free DNA: Proceed, but with Caution.J Am Soc Nephrol. 2020 Oct;31(10):2491-2492. doi: 10.1681/ASN.2020060915. Epub 2020 Aug 10. J Am Soc Nephrol. 2020. PMID: 32778533 Free PMC article. No abstract available.
References
-
- Gielis EM, Ledeganck KJ, De Winter BY, Del Favero J, Bosmans JL, Claas FH, Abramowicz D, Eikmans M: Cell-free DNA: An upcoming biomarker in transplantation. Am J Transplant 15: 2541–2551, 2015 - PubMed
-
- Lo YM, Tein MS, Pang CC, Yeung CK, Tong KL, Hjelm NM: Presence of donor-specific DNA in plasma of kidney and liver-transplant recipients. Lancet 351: 1329–1330, 1998 - PubMed
-
- Beck J, Bierau S, Balzer S, Andag R, Kanzow P, Schmitz J, Gaedcke J, Moerer O, Slotta JE, Walson P, Kollmar O, Oellerich M, Schütz E: Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin Chem 59: 1732–1741, 2013 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

