Hydrogen sulfide improves intestinal recovery following ischemia by endothelial nitric oxide-dependent mechanisms
- PMID: 28280145
- PMCID: PMC5451562
- DOI: 10.1152/ajpgi.00444.2016
Hydrogen sulfide improves intestinal recovery following ischemia by endothelial nitric oxide-dependent mechanisms
Abstract
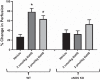
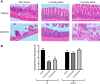
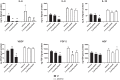
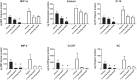
Hydrogen sulfide (H2S) is an endogenous gasotransmitter that has vasodilatory properties. It may be a novel therapy for intestinal ischemia-reperfusion (I/R) injury. We hypothesized that 1) H2S would improve postischemic survival, mesenteric perfusion, mucosal injury, and inflammation compared with vehicle and 2) the benefits of H2S would be mediated through endothelial nitric oxide. C57BL/6J wild-type and endothelial nitric oxide synthase knockout (eNOS KO) mice were anesthetized, and a midline laparotomy was performed. Intestines were eviscerated, the small bowel mesenteric root identified, and baseline intestinal perfusion was determined using laser Doppler. Intestinal ischemia was established by temporarily occluding the superior mesenteric artery. Following ischemia, the clamp was removed, and the intestines were allowed to recover. Either sodium hydrosulfide (2 nmol/kg or 2 µmol/kg NaHS) in PBS vehicle or vehicle only was injected into the peritoneum. Animals were allowed to recover and were assessed for mesenteric perfusion, mucosal injury, and intestinal cytokines. P values < 0.05 were significant. H2S improved mesenteric perfusion and mucosal injury scores following I/R injury. However, in the setting of eNOS ablation, there was no improvement in these parameters with H2S therapy. Application of H2S also resulted in lower levels of intestinal cytokine production following I/R. Intraperitoneal H2S therapy can improve mesenteric perfusion, intestinal mucosal injury, and intestinal inflammation following I/R. The benefits of H2S appear to be mediated through endothelial nitric oxide-dependent pathways.NEW & NOTEWORTHY H2S is a gaseous mediator that acts as an anti-inflammatory agent contributing to gastrointestinal mucosal defense. It promotes vascular dilation, mucosal repair, and resolution of inflammation following intestinal ischemia and may be exploited as a novel therapeutic agent. It is unclear whether H2S works through nitric oxide-dependent pathways in the intestine. We appreciate that H2S was able to improve postischemic recovery of mesenteric perfusion, mucosal integrity, and inflammation. The beneficial effects of H2S appear to be mediated through endothelial nitric oxide-dependent pathways.
Keywords: endothelial nitric oxide synthase; hydrogen sulfide; inflammation; intestinal ischemia; nitric oxide; perfusion; sodium hydrosulfide.
Copyright © 2017 the American Physiological Society.
Figures
References
-
- Blackler RW, De Palma G, Manko A, Da Silva GJ, Flannigan KL, Bercik P, Surette MG, Buret AG, Wallace JL. Deciphering the pathogenesis of NSAID enteropathy using proton pump inhibitors and a hydrogen sulfide-releasing NSAID. Am J Physiol Gastrointest Liver Physiol 308: G994–G1003, 2015. doi: 10.1152/ajpgi.00066.2015. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

