89Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection
- PMID: 28280216
- PMCID: PMC5577625
- DOI: 10.2967/jnumed.116.187310
89Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection
Abstract
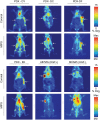
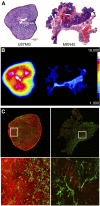
The hepatocyte growth factor (HGF) binding antibody rilotumumab (AMG102) was modified for use as a 89Zr-based immuno-PET imaging agent to noninvasively determine the local levels of HGF protein in tumors. Because recent clinical trials of HGF-targeting therapies have been largely unsuccessful in several different cancers (e.g., gastric, brain, lung), we have synthesized and validated 89Zr-DFO-AMG102 as a companion diagnostic for improved identification and selection of patients having high local levels of HGF in tumors. To date, patient selection has not been performed using the local levels of HGF protein in tumors. Methods: The chelator p-SCN-Bn-DFO was conjugated to AMG102, radiolabeling with 89Zr was performed in high radiochemical yields and purity (>99%), and binding affinity of the modified antibody was confirmed using an enzyme-linked immunosorbent assay (ELISA)-type binding assay. PET imaging, biodistribution, autoradiography and immunohistochemistry, and ex vivo HGF ELISA experiments were performed on murine xenografts of U87MG (HGF-positive, MET-positive) and MKN45 (HGF-negative, MET-positive) and 4 patient-derived xenografts (MET-positive, HGF unknown). Results: Tumor uptake of 89Zr-DFO-AMG102 at 120 h after injection in U87MG xenografts (HGF-positive) was high (36.8 ± 7.8 percentage injected dose per gram [%ID/g]), whereas uptake in MKN45 xenografts (HGF-negative) was 5.0 ± 1.3 %ID/g and a control of nonspecific human IgG 89Zr-DFO-IgG in U87MG tumors was 11.5 ± 3.3 %ID/g, demonstrating selective uptake in HGF-positive tumors. Similar experiments performed in 4 different gastric cancer patient-derived xenograft models showed low uptake of 89Zr-DFO-AMG102 (∼4-7 %ID/g), which corresponded with low HGF levels in these tumors (ex vivo ELISA). Autoradiography, immunohistochemical staining, and HGF ELISA assays confirmed that elevated levels of HGF protein were present only in U87MG tumors and that 89Zr-DFO-AMG102 uptake was closely correlated with HGF protein levels in tumors. Conclusion: The new immuno-PET imaging agent 89Zr-DFO-AMG102 was successfully synthesized, radiolabeled, and validated in vitro and in vivo to selectively accumulate in tumors with high local levels of HGF protein. These results suggest that 89Zr-DFO-AMG102 would be a valuable companion diagnostic tool for the noninvasive selection of patients with elevated local concentrations of HGF in tumors for planning any HGF-targeted therapy, with the potential to improve clinical outcomes.
Keywords: AMG102; HGF; MET; PET; hepatocyte growth factor; patient-derived xenograft; rilotumumab; scatter factor.
© 2017 by the Society of Nuclear Medicine and Molecular Imaging.
Figures





References
-
- Bottaro DP, Rubin J, Faletto D, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. - PubMed
-
- Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hematol. 2005;53:35–69. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
