Assessing the cost burden of United States FDA-mandated post-approval studies for medical devices
- PMID: 28280294
- PMCID: PMC5340422
Assessing the cost burden of United States FDA-mandated post-approval studies for medical devices
Abstract
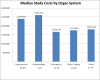
Approved medical devices frequently undergo FDA mandated post-approval studies (PAS). However, there is uncertainty as to the value of PAS in assessing the safety of medical devices and the cost of these studies to the healthcare system is unknown. Since PAS costs are funded through device manufacturers who do not share the costs with regulators, we sought to estimate the total PAS costs through interviews with a panel of experts in medical device clinical trial design in order to design a general cost model for PAS which was then applied to the FDA PAS. A total of 277 PAS were initiated between 3/1/05 through 6/30/13 and demonstrated a median cost of $2.16 million per study and an overall cost of $1.22 billion over the 8.25 years of study. While these costs are funded through manufacturers, the ultimate cost is borne by the healthcare system through the medical device costs. Given concerns regarding the informational value of PAS, the resources used to support mandated PAS may be better allocated to other approaches to assure safety.
Figures
References
-
- Resnic FS, Normand SL. Postmarketing surveillance of medical devices--filling in the gaps. The New England journal of medicine. 2012;366(10):875–7. - PubMed
-
- Reynolds IS, Rising JP, Coukell AJ, Paulson KH, Redberg RF. Assessing the safety and effectiveness of devices after US Food and Drug Administration approval: FDA-mandated postapproval studies. JAMA internal medicine. 2014;174(11):1773–9. - PubMed
-
- Kaplan AV, Baim DS, Smith JJ, Feigal DA, Simons M, Jefferys D, et al. Medical device development: from prototype to regulatory approval. Circulation. 2004;109(25):3068–72. - PubMed
-
- Daniel GW, McClellan MB, Colvin H, Aurora P, Khaterzai S. [May 10, 2015];Strengthening Patient Care: Building an Effective National Medical Device Surveillance System. http://www.brookings.edu/research/papers/2015/02/23-medical-device-postm....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources