Systemic and immunotoxicity of pristine and PEGylated multi-walled carbon nanotubes in an intravenous 28 days repeated dose toxicity study
- PMID: 28280324
- PMCID: PMC5339008
- DOI: 10.2147/IJN.S123345
Systemic and immunotoxicity of pristine and PEGylated multi-walled carbon nanotubes in an intravenous 28 days repeated dose toxicity study
Abstract
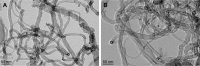
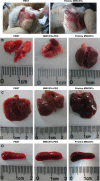
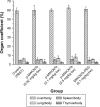
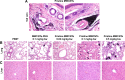
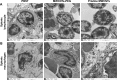
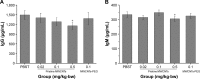
The numerous increasing use of carbon nanotubes (CNTs) derived from nanotechnology has raised concerns about their biosafety and potential toxicity. CNTs cause immunologic dysfunction and limit the application of CNTs in biomedicine. The immunological responses induced by pristine multi-walled carbon nanotubes (p-MWCNTs) and PEGylated multi-walled carbon nanotubes (MWCNTs-PEG) on BALB/c mice via an intravenous administration were investigated. The results reflect that the p-MWCNTs induced significant increases in spleen, thymus, and lung weight. Mice treated with p-MWCNTs showed altered lymphocyte populations (CD3+, CD4+, CD8+, and CD19+) in peripheral blood and increased serum IgM and IgG levels, and splenic macrophage ultrastructure indicated mitochondria swelling. p-MWCNTs inhibited humoral and cellular immunity function and were associated with decreased immune responses against sheep erythrocytes and serum hemolysis level. Natural killer (NK) activity was not modified by two types of MWCNTs. In comparison with two types of MWCNTs, for a same dose, p-MWCNTs caused higher levels of inflammation and immunosuppression than MWCNTs-PEG. The results of immunological function suggested that after intravenous administration with p-MWCNTs caused more damage to systemic immunity than MWCNTs-PEG. Here, we demonstrated that a surface functional modification on MWCNTs reduces their immune perturbations in vivo. The chemistry-modified MWCNTs change their preferred immune response in vivo and reduce the immunotoxicity of p-MWCNTs.
Keywords: BALB/c mice; immunosuppression; immunotoxicity; multi-walled carbon nanotubes; surface-functionalized.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Ajayan PM, Tour JM. Materials science: nanotube composites. Nature. 2007;447(7148):1066–1068. - PubMed
-
- Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4(10):627–633. - PubMed
-
- Laverny G, Casset A, Purohit A, et al. Immunomodulatory properties of multi-walled carbon nanotubes in peripheral blood mononuclear cells from healthy subjects and allergic patients. Toxicol Lett. 2013;217(2):91–101. - PubMed
-
- Wu HC, Chang X, Liu L, Zhao F, Zhao Y. Chemistry of carbon nanotubes in biomedical applications. J Mater Chem. 2010;20(6):1036–1052.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

