Tapentadol extended release for the management of chronic neck pain
- PMID: 28280384
- PMCID: PMC5338932
- DOI: 10.2147/JPR.S129056
Tapentadol extended release for the management of chronic neck pain
Abstract
Background: The role of opioids in the management of chronic neck pain is still poorly investigated. No data are available on tapentadol extended release (ER). In this article, we present 54 patients with moderate-to-severe chronic neck pain treated with tapentadol ER.
Patients and methods: Patients received tapentadol ER 100 mg/day; dosage was then adjusted according to clinical needs. The following parameters were recorded: pain; Douleur Neuropathique 4 score; Neck Disability Index score; range of motion; pain-associated sleep interference; quality of life (Short Form [36] Health Survey); Patient Global Impression of Change (PGIC); Clinician GIC; opioid-related adverse effects; and need for other analgesics.
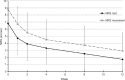
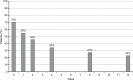
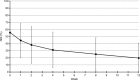
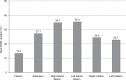
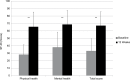
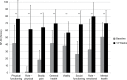
Results: A total of 44 of 54 patients completed the 12-week observation. Tapentadol ER daily doses increased from 100 mg/day to a mean (standard deviation) dosage of 204.5 (102.8) mg/day at the final evaluation. Mean pain intensity at movement significantly decreased from baseline (8.1 [1.1]) to all time points (P<0.01). At baseline, 70% of patients presented a positive neuropathic component. This percentage dropped to 23% after 12 weeks. Tapentadol improved Neck Disability Index scores from 55.6 (18.6) at baseline to 19.7 (20.9) at the final evaluation (P<0.01). Tapentadol significantly improved neck range of motion in all three planes of motion, particularly in lateral flexion. Quality of life significantly improved in all Short Form (36) Health Survey subscales (P<0.01) and in both physical and mental status (P<0.01). Based on PGIC results, approximately 90% of patients rated their overall condition as much/very much improved. Tapentadol was well tolerated: no patients discontinued due to side effects. The use of other analgesics was reduced during the observed period.
Conclusion: Our results suggest that tapentadol ER, started at 100 mg/day, is effective and well tolerated in patients with moderate-to-severe chronic neck pain, including opioid-naïve subjects. Patients can expect a decrease in pain, an improvement in neck function, and a decrease in neuropathic symptoms.
Keywords: Neck Disability Index; chronic neck pain; neuropathic pain; opioids; range of motion; tapentadol.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Tapentadol prolonged release for patients with multiple myeloma suffering from moderate-to-severe cancer pain due to bone disease.J Pain Res. 2015 May 8;8:229-38. doi: 10.2147/JPR.S83490. eCollection 2015. J Pain Res. 2015. PMID: 26064064 Free PMC article.
-
Tapentadol prolonged release for managing moderate to severe chronic neck pain with or without a neuropathic component.Curr Med Res Opin. 2020 Apr;36(4):651-659. doi: 10.1080/03007995.2020.1722083. Epub 2020 Feb 6. Curr Med Res Opin. 2020. PMID: 31983248
-
Effectiveness and safety of tapentadol prolonged release for severe, chronic low back pain with or without a neuropathic pain component: results of an open-label, phase 3b study.Curr Med Res Opin. 2012 Jun;28(6):911-36. doi: 10.1185/03007995.2012.679254. Epub 2012 May 9. Curr Med Res Opin. 2012. PMID: 22443293 Clinical Trial.
-
Efficacy of tapentadol ER for managing moderate to severe chronic pain.Pain Physician. 2013 Jan;16(1):27-40. Pain Physician. 2013. PMID: 23340531 Review.
-
Tapentadol Prolonged Release for Chronic Pain: A Review of Clinical Trials and 5 Years of Routine Clinical Practice Data.Pain Pract. 2017 Jun;17(5):678-700. doi: 10.1111/papr.12515. Epub 2016 Oct 25. Pain Pract. 2017. PMID: 27611642 Review.
Cited by
-
Opioid system and related ligands: from the past to future perspectives.J Anesth Analg Crit Care. 2024 Oct 11;4(1):70. doi: 10.1186/s44158-024-00201-2. J Anesth Analg Crit Care. 2024. PMID: 39390585 Free PMC article. Review.
-
Tapentadol: an effective option for the treatment of back pain.J Pain Res. 2019 May 16;12:1521-1528. doi: 10.2147/JPR.S190176. eCollection 2019. J Pain Res. 2019. PMID: 31190963 Free PMC article. Review.
-
The Challenge of Managing Neuropathic Pain in Children and Adolescents with Cancer.Cancers (Basel). 2025 Jan 29;17(3):460. doi: 10.3390/cancers17030460. Cancers (Basel). 2025. PMID: 39941827 Free PMC article. Review.
-
Single intracutaneous injection of local anesthetics and steroids alleviates acute nonspecific neck pain: A CONSORT-perspective, randomized, controlled clinical trial.Medicine (Baltimore). 2018 Jul;97(28):e11285. doi: 10.1097/MD.0000000000011285. Medicine (Baltimore). 2018. PMID: 29995761 Free PMC article. Clinical Trial.
-
Pain reduction induced by tapentadol in patients with musculoskeletal chronic pain fosters better sleep quality.Drugs Context. 2021 Apr 19;10:2020-12-9. doi: 10.7573/dic.2020-12-9. eCollection 2021. Drugs Context. 2021. PMID: 33953781 Free PMC article.
References
-
- Hoy DG, Protani M, De R, Buchbinder R. The epidemiology of neck pain. Best Pract Res Rheumatol. 2010;24(6):783–792. - PubMed
-
- Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33(4 Suppl):S39–S51. - PubMed
-
- Teichtahl AJ, McColl G. An approach to neck pain for family physicians. Aust Fam Physician. 2013;42(11):774–777. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

