The Role of Glia in the Peripheral and Central Auditory System Following Noise Overexposure: Contribution of TNF-α and IL-1β to the Pathogenesis of Hearing Loss
- PMID: 28280462
- PMCID: PMC5322242
- DOI: 10.3389/fnana.2017.00009
The Role of Glia in the Peripheral and Central Auditory System Following Noise Overexposure: Contribution of TNF-α and IL-1β to the Pathogenesis of Hearing Loss
Abstract
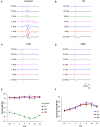
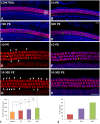
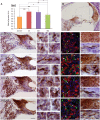
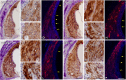
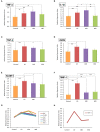
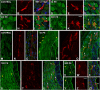
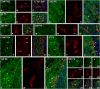
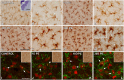
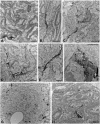
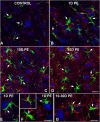
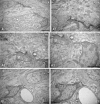
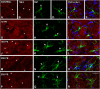
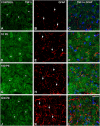
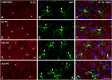
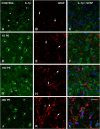
Repeated noise exposure induces inflammation and cellular adaptations in the peripheral and central auditory system resulting in pathophysiology of hearing loss. In this study, we analyzed the mechanisms by which noise-induced inflammatory-related events in the cochlea activate glial-mediated cellular responses in the cochlear nucleus (CN), the first relay station of the auditory pathway. The auditory function, glial activation, modifications in gene expression and protein levels of inflammatory mediators and ultrastructural changes in glial-neuronal interactions were assessed in rats exposed to broadband noise (0.5-32 kHz, 118 dB SPL) for 4 h/day during 4 consecutive days to induce long-lasting hearing damage. Noise-exposed rats developed a permanent threshold shift which was associated with hair cell loss and reactive glia. Noise-induced microglial activation peaked in the cochlea between 1 and 10D post-lesion; their activation in the CN was more prolonged reaching maximum levels at 30D post-exposure. RT-PCR analyses of inflammatory-related genes expression in the cochlea demonstrated significant increases in the mRNA expression levels of pro- and anti-inflammatory cytokines, inducible nitric oxide synthase, intercellular adhesion molecule and tissue inhibitor of metalloproteinase-1 at 1 and 10D post-exposure. In noise-exposed cochleae, interleukin-1β (IL-1β), and tumor necrosis factor α (TNF-α) were upregulated by reactive microglia, fibrocytes, and neurons at all time points examined. In the CN, however, neurons were the sole source of these cytokines. These observations suggest that noise exposure causes peripheral and central inflammatory reactions in which TNF-α and IL-1β are implicated in regulating the initiation and progression of noise-induced hearing loss.
Keywords: auditory system; cochlear nucleus; cytokines; inflammation; inner ear.
Figures
Similar articles
-
The TLR-4/NF-κB signaling pathway activation in cochlear inflammation of rats with noise-induced hearing loss.Hear Res. 2019 Aug;379:59-68. doi: 10.1016/j.heares.2019.04.012. Epub 2019 May 3. Hear Res. 2019. PMID: 31096078
-
Proinflammatory cytokines expression in noise-induced damaged cochlea.J Neurosci Res. 2006 Mar;83(4):575-83. doi: 10.1002/jnr.20764. J Neurosci Res. 2006. PMID: 16429448
-
The circadian timing of noise exposure influences noise-induced inflammatory responses in the mouse cochlea.Braz J Otorhinolaryngol. 2022 Nov-Dec;88 Suppl 3(Suppl 3):S1-S8. doi: 10.1016/j.bjorl.2021.05.010. Epub 2021 Jun 12. Braz J Otorhinolaryngol. 2022. PMID: 34217678 Free PMC article.
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
-
[Central and peripheral aspects of noise-induced hearing loss].HNO. 2018 May;66(5):342-349. doi: 10.1007/s00106-017-0442-9. HNO. 2018. PMID: 29204664 Review. German.
Cited by
-
Microglia Regulate Pruning of Specialized Synapses in the Auditory Brainstem.Front Neural Circuits. 2019 Aug 28;13:55. doi: 10.3389/fncir.2019.00055. eCollection 2019. Front Neural Circuits. 2019. PMID: 31555101 Free PMC article.
-
Engraftment of Human Stem Cell-Derived Otic Progenitors in the Damaged Cochlea.Mol Ther. 2019 Jun 5;27(6):1101-1113. doi: 10.1016/j.ymthe.2019.03.018. Epub 2019 Apr 2. Mol Ther. 2019. PMID: 31005598 Free PMC article.
-
Auditory Brainstem Deficits from Early Treatment with a CSF1R Inhibitor Largely Recover with Microglial Repopulation.eNeuro. 2021 Mar 22;8(2):ENEURO.0318-20.2021. doi: 10.1523/ENEURO.0318-20.2021. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33558268 Free PMC article.
-
Tumor necrosis factor-α mediated inflammation versus apoptosis in age-related hearing loss.Front Aging Neurosci. 2022 Sep 7;14:956503. doi: 10.3389/fnagi.2022.956503. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36158549 Free PMC article.
-
Multiplex immunohistochemistry reveals cochlear macrophage heterogeneity and local auditory nerve inflammation in cisplatin-induced hearing loss.Front Neurol. 2022 Oct 20;13:1015014. doi: 10.3389/fneur.2022.1015014. eCollection 2022. Front Neurol. 2022. PMID: 36341090 Free PMC article.
References
-
- Alvarado J. C., Fuentes-Santamaría V., Gabaldón-Ull M. C., Jareño-Flores T., Miller J. M., Juiz J. M. (2016). Noise-induced “toughening” effect in wistar rats: enhanced auditory brainstem responses are related to calretinin and nitric oxide synthase upregulation. Front. Neuroanat. 10:19. 10.3389/fnana.2016.00019 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

