Reactive Oxygen Species Mediated Prostaglandin E2 Contributes to Acute Response of Epithelial Injury
- PMID: 28280524
- PMCID: PMC5322452
- DOI: 10.1155/2017/4123854
Reactive Oxygen Species Mediated Prostaglandin E2 Contributes to Acute Response of Epithelial Injury
Abstract
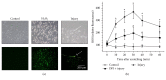
Reactive oxygen species (ROS) generated after tissue injury play a crucial role during wound healing through initiating acute inflammation, clarifying infection and dead tissue, and mediating various intracellular signal transduction. Prostaglandin E2 (PGE2) has been identified as one of the major factors responsible for inflammation and tissue repair. In this study, we tested our hypothesis that ROS produced by damaged human keratinocytes induces the synthesis of PGE2. In vitro epithelial wounding model was used to observe the production of ROS and secretion of PGE2 as well as the involved signal pathway. The mechanical injury caused the rapid production of ROS in in vitro cultured keratinocytes, which was significantly blocked by an inhibitor of nicotinamide adenine dinucleotide phosphate oxidase. The increased intracellular ROS caused by mechanical injury stimulates PGE2 production in a time-dependent manner via the activation of cyclooxygenase-2 (COX-2), which was stimulated by phosphorylation of extracellular signal-regulated protein kinase (ERK). These results indicate ROS-induced ERK activation leading to the activation of COX-2 and the synthesis of PGE2 in human keratinocytes responding to mechanical injury in the acute phase.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

