Evaluation of two endometriosis models by transplantation of human endometrial tissue fragments and human endometrial mesenchymal cells
- PMID: 28280797
- PMCID: PMC5340136
Evaluation of two endometriosis models by transplantation of human endometrial tissue fragments and human endometrial mesenchymal cells
Abstract
Background: The animal models of endometriosis could be a valuable alternative tool for clarifying the etiology of endometriosis.
Objective: In this study two endometriosis models at the morphological and molecular levels was evaluated and compared.
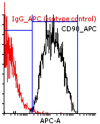
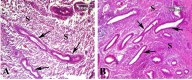
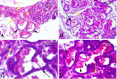
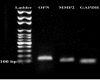
Materials and methods: The human endometrial tissues were cut into small fragments then they were randomly considered for transplantation into γ irradiated mice as model A; or they were isolated and cultured up to fourth passages. 2×106 cultured stromal cells were transplanted into γ irradiated mice subcutaneously as model B. twenty days later the ectopic tissues in both models were studied morphologically by Periodic acid-Schiff and hematoxylin and eosin staining. The expression of osteopontin (OPN) and matrix metalloproteinase 2 (MMP2) genes were also assessed using real time RT-PCR. 17-β estradiol levels of mice sera were compared before and after transplantation.
Results: The endometrial like glands and stromal cells were formed in the implanted subcutaneous tissue of both endometriosis models. The gland sections per cubic millimeter, the expression of OPN and MMP2 genes and the level of 17-β estradiol were higher in model B than model A (p=0.03).
Conclusion: Our observation demonstrated that endometrial mesenchymal stromal cells showed more efficiency to establish endometriosis model than human endometrial tissue fragments.
Keywords: Endometriosis; Matrix metalloproteinase 2; Osteopontin; Stromal cells.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. None of the authors are directly funded by or employed by the Government of Iran.
Figures





Similar articles
-
Osteopontin Regulates Endometrial Stromal Cell Migration in Endometriosis through the PI3K Pathway : Osteopontin Regulates Endometrial Cell Migration in Endometriosis.Reprod Sci. 2021 Feb;28(2):435-446. doi: 10.1007/s43032-020-00301-8. Epub 2020 Sep 9. Reprod Sci. 2021. PMID: 32909189 Free PMC article.
-
The involvement of osteopontin and matrix metalloproteinase- 9 in the migration of endometrial epithelial cells in patients with endometriosis.Reprod Biol Endocrinol. 2015 Aug 20;13:95. doi: 10.1186/s12958-015-0090-4. Reprod Biol Endocrinol. 2015. PMID: 26289107 Free PMC article.
-
Human chorionic gonadotropin induces decidualization of ectopic human endometrium more effectively than forskolin in an in-vivo endometriosis model.Exp Biol Med (Maywood). 2018 Jul;243(11):953-962. doi: 10.1177/1535370218782658. Epub 2018 Jun 9. Exp Biol Med (Maywood). 2018. PMID: 29886768 Free PMC article.
-
The expression and significance of leukemia inhibitory factor, interleukin-6 and vascular endothelial growth factor in Chinese patients with endometriosis.Arch Gynecol Obstet. 2021 Jul;304(1):163-170. doi: 10.1007/s00404-021-05980-5. Epub 2021 Feb 8. Arch Gynecol Obstet. 2021. PMID: 33555431
-
Estrogen- and Progesterone (P4)-Mediated Epigenetic Modifications of Endometrial Stromal Cells (EnSCs) and/or Mesenchymal Stem/Stromal Cells (MSCs) in the Etiopathogenesis of Endometriosis.Stem Cell Rev Rep. 2021 Aug;17(4):1174-1193. doi: 10.1007/s12015-020-10115-5. Epub 2021 Jan 7. Stem Cell Rev Rep. 2021. PMID: 33411206 Free PMC article. Review.
Cited by
-
The long road of drug development for endometriosis - Pains, gains, and hopes.J Control Release. 2024 Dec;376:429-440. doi: 10.1016/j.jconrel.2024.10.036. Epub 2024 Oct 24. J Control Release. 2024. PMID: 39427778 Free PMC article. Review.
-
Mesenchymal Stromal Cells Are More Immunosuppressive In Vitro If They Are Derived from Endometriotic Lesions than from Eutopic Endometrium.Stem Cells Int. 2017;2017:3215962. doi: 10.1155/2017/3215962. Epub 2017 Nov 5. Stem Cells Int. 2017. PMID: 29230250 Free PMC article.
-
Effect of PLGA Nanoparticle-Mediated Delivery of miRNA 503 on The Apoptosis of Ovarian Endometriosis Cells.Cell J. 2022 Nov 2;24(11):697-704. doi: 10.22074/cellj.2022.557554.1069. Cell J. 2022. PMID: 36377220 Free PMC article.
-
Rosiglitazone affects the progression of surgically‑induced endometriosis in a rat model.Mol Med Rep. 2021 Jan;23(1):35. doi: 10.3892/mmr.2020.11673. Epub 2020 Nov 12. Mol Med Rep. 2021. PMID: 33179107 Free PMC article.
-
It Is Necessary to Purpose an Add-on to the American Classification of Endometriosis? This Disease Can Be Compared to a Malignant Proliferation While Remaining Benign in Most Cases. EndoGram® Is a New Profile Witness of Its Evolutionary Potential.Front Surg. 2019 Jun 7;6:27. doi: 10.3389/fsurg.2019.00027. eCollection 2019. Front Surg. 2019. PMID: 31231658 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
