A new media without animal component for sperm cryopreservation: motility and various attributes affecting paternal contribution of sperm
- PMID: 28281145
- PMCID: PMC5427647
- DOI: 10.1007/s10815-017-0888-4
A new media without animal component for sperm cryopreservation: motility and various attributes affecting paternal contribution of sperm
Abstract
Purpose: Our aim was the development of a safe sperm cryopreservation New Media (NM), composed of consistent and reproducible components devoid of any animal origin, and evaluation of NM in terms of its effect on sperm structure and function as compared to regularly used yolk media (TYM) (Irvine Scientific).
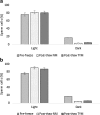
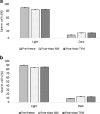
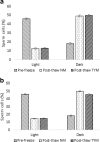
Methods: We evaluated patient semen samples and cryopreserved them in duplicates in either NM or TYM. The samples were cryopreserved for either a short term of 1 week or long term of 1 month prior to thawing. The parameters investigated include sperm motility via computer-assisted semen analysis (CASA), sperm concentration, and sperm biomarkers that promote paternal contribution of spermatozoa to fertilization including hyaluronic acid binding, chromatin maturity, apoptotic markers, cytoplasmic retention, and sperm DNA integrity.
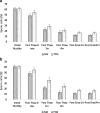
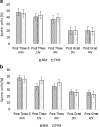
Results: As compared to TYM, NM was equally capable of sperm cryopreservation with both short-term and long-term storage in media, and after freeze-thaw and gradient processing of sperm. HA binding of sperm was comparable post thaw in both NM and yolk media. There are also no differences observed between the samples cryopreserved in NM or TYM in terms of their aniline blue staining, CK immunocytochemistry, caspase 3 immunostaining, or DNA nick translation.
Conclusions: NM has the advantage of being xeno-free, yet in preservation of sperm motility and other sperm attributes, the NM is as effective as the TYM.
Keywords: Biochemical markers; Cryopreservation; HA binding; Male fertility; Sperm maturity; Sperm motility.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest. Funding for the research was provided by VitroLife for evaluation of New Media.
Ethical approval
The study was approved by the Yale University IRB.
Informed consent
Informed consent was obtained from all individual participants included in the study. The patient sperm samples were de-identified prior to the initiation of study.
Figures



References
-
- Rosen A. Third-party reproduction and adoption in cancer patients. J Natl Cancer Inst Monogr. 2005;(34):91–3. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

