Structural basis of homo- and heterotrimerization of collagen I
- PMID: 28281531
- PMCID: PMC5353611
- DOI: 10.1038/ncomms14671
Structural basis of homo- and heterotrimerization of collagen I
Abstract
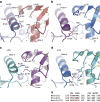
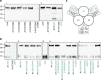
Fibrillar collagen molecules are synthesized as precursors, procollagens, with large propeptide extensions. While a homotrimeric form (three α1 chains) has been reported in embryonic tissues as well as in diseases (cancer, fibrosis, genetic disorders), collagen type I usually occurs as a heterotrimer (two α1 chains and one α2 chain). Inside the cell, the role of the C-terminal propeptides is to gather together the correct combination of three α chains during molecular assembly, but how this occurs for different forms of the same collagen type is so far unknown. Here, by structural and mutagenic analysis, we identify key amino acid residues in the α1 and α2 C-propeptides that determine homo- and heterotrimerization. A naturally occurring mutation in one of these alters the homo/heterotrimer balance. These results show how the C-propeptide of the α2 chain has specifically evolved to permit the appearance of heterotrimeric collagen I, the major extracellular building block among the metazoa.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
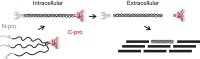
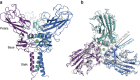
References
-
- Uitto J. Collagen polymorphism: isolation and partial characterization of α1(I)-trimer molecules in normal human skin. Arch. Biochem. Biophys. 192, 371–379 (1979). - PubMed
-
- Jimenez S. A., Bashey R. I., Benditt M. & Yankowski R. Identification of collagen α1(I) trimer in embryonic chick tendons and calvaria. Biochem. Biophys. Res. Commun. 78, 1354–1361 (1977). - PubMed
-
- Sengupta P. K., Smith E. M., Kim K., Murnane M. J. & Smith B. D. DNA hypermethylation near the transcription start site of collagen α2(I) gene occurs in both cancer cell lines and primary colorectal cancers. Cancer Res. 63, 1789–1797 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

