Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson's disease
- PMID: 28281653
- PMCID: PMC5345073
- DOI: 10.1038/srep44373
Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson's disease
Abstract
Therapeutic targets are needed to develop neuroprotective treatments for Parkinson's disease (PD). Mitophagy, the selective autophagic elimination of dysfunctional mitochondria, is essential for the maintenance of mitochondrial integrity and is predominantly regulated by the PINK1/Parkin-mediated pathway. Loss of function mutations in Parkin and PINK1 cause an accumulation of dysfunctional mitochondria, leading to nigral neurodegeneration and early-onset PD with a high penetrance rate. We previously identified an asymptomatic homozygous Parkin mutation carrier who had not developed PD by her eighth decade despite the loss of functional Parkin. Here we discover a putative mechanism that protects her against PD. In contrast to Parkin-related PD patient-derived cells, the asymptomatic carrier cells show preserved mitochondrial function and mitophagy which is mediated by mitochondrial receptor Nip3-like protein X (Nix). Nix-mediated mitophagy was not affected by PINK1 knockdown. Both genetic and pharmacological induction of Nix restores mitophagy in PINK1- and Parkin-related PD patient cell lines, confirming its ability to induce mitophagy in the absence of PINK1/Parkin-mediated pathway. Moreover, Nix over-expression improves mitochondrial ATP production in these patient cells. Our results demonstrate that Nix can serve as an alternative mediator of mitophagy to maintain mitochondrial turnover, identifying Nix as a promising target for neuroprotective treatment in PINK1/Parkin-related PD.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


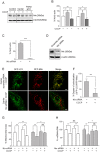
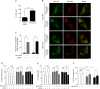

Comment in
-
Nature's Parkin experiment: Nix-a novel protective mechanism in Parkinson's disease.Mov Disord. 2017 Jul;32(7):992. doi: 10.1002/mds.27015. Epub 2017 Apr 22. Mov Disord. 2017. PMID: 28432765 No abstract available.
-
Commentary: Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson's disease.Front Mol Neurosci. 2017 Sep 19;10:297. doi: 10.3389/fnmol.2017.00297. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28974925 Free PMC article. No abstract available.
References
-
- Ryan B. J., Hoek S., Fon E. A. & Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci 40, 200–210 (2015). - PubMed
-
- Corti O., Lesage S. & Brice A. What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev 91, 1161–1218 (2011). - PubMed
-
- Hirsch E. C., Jenner P. & Przedborski S. Pathogenesis of Parkinson’s disease. Mov Disord 28, 24–30 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

