Vaccines licensed and in clinical trials for the prevention of dengue
- PMID: 28281864
- PMCID: PMC5443395
- DOI: 10.1080/21645515.2016.1261770
Vaccines licensed and in clinical trials for the prevention of dengue
Abstract
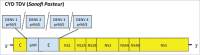
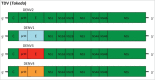
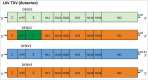
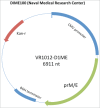
Dengue has become a major global public health threat with almost half of the world's population living in at-risk areas. Vaccination would likely represent an effective strategy for the management of dengue disease in endemic regions, however to date there is only one licensed preventative vaccine for dengue infection. The development of a vaccine against dengue virus (DENV) has been hampered by an incomplete understanding of protective immune responses against DENV. The most clinically advanced dengue vaccine is the chimeric yellow fever-dengue vaccine (CYD) that employs the yellow fever virus 17D strain as the replication backbone (Chimerivax-DEN; CYD-TDV). This vaccine had an overall pooled protective efficacy of 65.6% but was substantially more effective against severe dengue and dengue hemorrhagic fever. Several other vaccine approaches have been developed including live attenuated chimeric dengue vaccines (DENVax and LAV Delta 30), DEN protein subunit V180 vaccine (DEN1-80E) and DENV DNA vaccines. These vaccines have been shown to be immunogenic in animals and also safe and immunogenic in humans. However, these vaccines are yet to progress to phase III trials to determine their protective efficacy against dengue. This review will summarize the details of vaccines that have progressed to clinical trials in humans.
Keywords: dengue; dengue vaccines; immune response; neutralising antibody; preventative vaccination.
Figures

References
-
- Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci U S A 2006; 103:6242-7; PMID:16606847; http://dx.doi.org/10.1073/pnas.0508391103 - DOI - PMC - PubMed
-
- World Health Organization 2012 Global strategy for dengue prevention and control.
-
- Tapia-Conyer R, Betancourt-Cravioto M, Mendez-Galvan J. Dengue: an escalating public health problem in Latin America. Paediatr Int Child Health 2012; 32 Suppl 1:14-7; PMID:22668444; http://dx.doi.org/10.1179/2046904712Z.00000000046 - DOI - PMC - PubMed
-
- Dantes HG, Farfan-Ale JA, Sarti E. Epidemiological trends of dengue disease in Mexico (2000-2011): a systematic literature search and analysis. PLoS Negl Trop Dis 2014; 8:e3158; PMID:25375162; http://dx.doi.org/10.1371/journal.pntd.0003158 - DOI - PMC - PubMed
-
- Bravo L, Roque VG, Brett J, Dizon R, L'Azou M. Epidemiology of dengue disease in the Philippines (2000-2011): a systematic literature review. PLoS Negl Trop Dis 2014; 8:e3027; PMID:25375119; http://dx.doi.org/10.1371/journal.pntd.0003027 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
