Distribution of pericellular matrix molecules in the temporomandibular joint and their chondroprotective effects against inflammation
- PMID: 28282029
- PMCID: PMC5379161
- DOI: 10.1038/ijos.2016.57
Distribution of pericellular matrix molecules in the temporomandibular joint and their chondroprotective effects against inflammation
Abstract
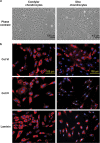
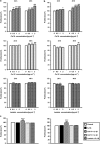
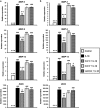
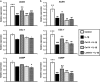
The objectives of this study were to (1) determine the distribution and synthesis of pericellular matrix (PCM) molecules (collagen VI, collagen IV and laminin) in rat temporomandibular joint (TMJ) and (2) investigate the effects of PCM molecules on chondrocytes against inflammation in osteoarthritis. Four zones (fibrous, proliferating, mature and hypertrophic) of condylar cartilage and three bands (anterior, intermediate and posterior) of disc were analysed by immunohistochemistry for the presence of PCM molecules in rat TMJs. Isolated chondrocytes were pre-treated with PCM molecules before being subjected to interleukin (IL)-1β treatment to stimulate inflammation. The responses of the chondrocytes were analysed using gene expression, nitric oxide release and matrix metalloproteinase (MMP)-13 production measures. Histomorphometric analyses revealed that the highest areal deposition of collagen VI (67.4%), collagen IV (45.7%) and laminin (52.4%) was in the proliferating zone of TMJ condylar cartilage. No significant difference in the distribution of PCM molecules was noted among the three bands of the TMJ disc. All three PCM molecules were expressed intracellularly by chondrocytes cultured in the monolayer. Among the PCM molecules, pre-treatment with collagen VI enhanced cellular proliferation, ameliorated IL-1β-induced MMP-3, MMP-9, MMP-13 and inducible nitric oxide synthase gene expression, and attenuated the downregulation of cartilage matrix genes, including collagen I, aggrecan and cartilage oligomeric matrix protein (COMP). Concurrently, collagen VI pretreatment inhibited nitric oxide and MMP-13 production. Our study demonstrates for the first time the distribution and role of PCM molecules, particularly collagen VI, in the protection of chondrocytes against inflammation.
Figures



References
-
- Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res 1987; 5 (4): 509–522. - PubMed
-
- Schminke B, Frese J, Bode C et al. Laminins and nidogens in the pericellular matrix of chondrocytes: their role in osteoarthritis and chondrogenic differentiation. Am J Pathol 2016; 186 (2): 410–418. - PubMed
-
- Guilak F, Alexopoulos LG, Upton ML et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci 2006; 1068: 498–512. - PubMed
-
- Hing WA, Sherwin AF, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthr Cartil 2002; 10 (4): 297–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

