Randomized phase 3 study of elotuzumab for relapsed or refractory multiple myeloma: ELOQUENT-2 Japanese patient subanalysis
- PMID: 28282035
- PMCID: PMC5380903
- DOI: 10.1038/bcj.2017.18
Randomized phase 3 study of elotuzumab for relapsed or refractory multiple myeloma: ELOQUENT-2 Japanese patient subanalysis
Conflict of interest statement
KSuz has received personal fees from Celgene, Janssen Pharmaceutical, Takeda and Novartis. KSun has received research funding from Ono Pharmaceutical Co. Ltd., Takeda, Celgene K.K., Novartis Pharma K.K., Bristol-Myers Squibb K.K., Daiichi Sankyo Co. Ltd., Sanofi K.K. and Janssen Pharmaceutical K.K., and lecture honoraria from Celgene K.K. KO has nothing to declare. SI has received research funding from Kyowa Hakko Kirin, Chugai, Takeda, Celgene, Ono Pharmaceutical Co. Ltd., Eli Lilly, Sanofi, Novartis, Teijin, Astellas, Toyama Chemical, Janssen Pharmaceutical and Bristol-Myers Squibb KK, and Bristol-Myers Squibb K.K. and honoraria from Celgene, Janssen, Ono Pharmaceutical Co. Ltd., Takeda and Bristol-Myers Squibb K.K. TM has received honoraria for lectures from Janssen Pharmaceutical, Celgene, Kyowa Hakko Kirin, Astellas and Chugai Pharmaceuticals, and research funding from Novartis. HH has received research funding from Bristol-Myers Squibb K.K., Kyowa Hakko Kirin, Nihon Shinyaku and Toyama Kagaku. KM has received honoraria from Celgene. MMiy is an employee of Bristol-Myers Squibb K.K. EB is an employee of Bristol-Myers Squibb. MMat has received honoraria from Janssen Pharmaceutical and Celgene. MMat has received research funding from Celgene, Bristol-Myers Squibb K.K. Kyowa Hakko Kirin, Chugai Pharma and Janssen Pharmaceutical.
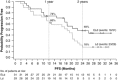
Figures
References
-
- Iida S, Nagai H, Kinoshita G, Miyoshi M, Robbins M, Pandya D et al. Elotuzumab with lenalidomide and dexamethasone for Japanese patients with relapsed/refractory multiple myeloma: phase 1 study. Int J Hematol 2017; 105: 326–334. - PubMed
-
- Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631. - PubMed
-
- Dimopoulos M, Lonial S, Casado LF, Golightly M, Doyen C, Shelat S et al. Elotuzumab: serum protein electrophoresis and immunofixation interference with clinical assessment of M-protein response in relapsed/refractory multiple myeloma (RRMM). Clin Lymphoma Myeloma Leuk 2015; 15: e267–e268.
-
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1782–1791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical