Enantioselective amine α-functionalization via palladium-catalysed C-H arylation of thioamides
- PMID: 28282045
- PMCID: PMC5347480
- DOI: 10.1038/nchem.2619
Enantioselective amine α-functionalization via palladium-catalysed C-H arylation of thioamides
Abstract
Saturated aza-heterocycles are highly privileged building blocks that are commonly encountered in bioactive compounds and approved therapeutic agents. These N-heterocycles are also incorporated as chiral auxiliaries and ligands in asymmetric synthesis. As such, the development of methods to functionalize the α-methylene C-H bonds of these systems enantioselectively is of great importance, especially in drug discovery. Currently, enantioselective lithiation with (-)-sparteine followed by Pd(0) catalysed cross-coupling to prepare α-arylated amines is largely limited to pyrrolidines. Here we report a Pd(II)-catalysed enantioselective α-C-H coupling of a wide range of amines, which include ethyl amines, azetidines, pyrrolidines, piperidines, azepanes, indolines and tetrahydroisoquinolines. Chiral phosphoric acids are demonstrated as effective anionic ligands for the enantioselective coupling of methylene C-H bonds with aryl boronic acids. This catalytic reaction not only affords high enantioselectivities, but also provides exclusive regioselectivity in the presence of two methylene groups in different steric environments.
Figures
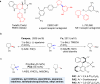
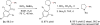
References
-
- Royer J. Asymmetric Synthesis of Nitrogen Heterocycles. Wiley-VCH; Weinheim, Germany: 2009.
-
- Nugent TC. Chiral Amine Synthesis: Methods, Developments and Applications. Wiley VCH; Weinheim, Germany: 2010.
-
- Campos KR. Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 2007;36:1069–1084. - PubMed
-
- Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 2012;18:10092–10142. - PubMed
-
- Beak P, Kerrick ST, Wu S, Chu J. Complex induced proximity effects: enantioselective syntheses based on asymmetric deprotonations of N-Boc-pyrrolidines. J. Am. Chem. Soc. 1994;116:3231–3239.
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/317226004
- PubChem-Substance/317226005
- PubChem-Substance/317226006
- PubChem-Substance/317226007
- PubChem-Substance/317226008
- PubChem-Substance/317226009
- PubChem-Substance/317226011
- PubChem-Substance/317226010
- PubChem-Substance/317226012
- PubChem-Substance/317226013
- PubChem-Substance/317226014
- PubChem-Substance/317226015
- PubChem-Substance/317226016
- PubChem-Substance/317226017
- PubChem-Substance/317226018
- PubChem-Substance/317226019
- PubChem-Substance/317226020
- PubChem-Substance/317226021
- PubChem-Substance/317226022
- PubChem-Substance/317226023
- PubChem-Substance/317226024
- PubChem-Substance/317226025
- PubChem-Substance/317226026
- PubChem-Substance/317226027
- PubChem-Substance/317226028
- PubChem-Substance/317226029
- PubChem-Substance/317226030
- PubChem-Substance/317226031
- PubChem-Substance/317226032
- PubChem-Substance/317226033
- PubChem-Substance/317226034
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

