Alterations in the Salivary Proteome and N-Glycome of Sjögren's Syndrome Patients
- PMID: 28282148
- PMCID: PMC9668345
- DOI: 10.1021/acs.jproteome.6b01051
Alterations in the Salivary Proteome and N-Glycome of Sjögren's Syndrome Patients
Abstract
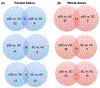
We used isobaric mass tagging (iTRAQ) and lectin affinity capture mass spectrometry (MS)-based workflows for global analyses of parotid saliva (PS) and whole saliva (WS) samples obtained from patients diagnosed with primary Sjögren's Syndrome (pSS) who were enrolled in the Sjögren's International Collaborative Clinical Alliance (SICCA) as compared with two control groups. The iTRAQ analyses revealed up- and down-regulation of numerous proteins that could be involved in the disease process (e.g., histones) or attempts to mitigate the ensuing damage (e.g., bactericidal/permeability increasing fold containing family (BPIF) members). An immunoblot approach applied to independent sample sets confirmed the pSS associated up-regulation of β2-microglobulin (in PS) and down-regulation of carbonic anhydrase VI (in WS) and BPIFB2 (in PS). Beyond the proteome, we profiled the N-glycosites of pSS and control samples. They were enriched for glycopeptides using lectins Aleuria aurantia and wheat germ agglutinin, which recognize fucose and sialic acid/N-acetyl glucosamine, respectively. MS analyses showed that pSS is associated with increased N-glycosylation of numerous salivary glycoproteins in PS and WS. The observed alterations of the salivary proteome and N-glycome could be used as pSS biomarkers enabling easier and earlier detection of this syndrome while lending potential new insights into the disease process.
Keywords: N-glycosylation; Sjögren’s Syndrome; glycosite; iTRAQ; isobaric mass tagging; lectin affinity capture; parotid saliva; whole saliva.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


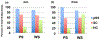
References
-
- Fox PC Autoimmune diseases and Sjogren’s syndrome: an autoimmune exocrinopathy. Ann. N. Y. Acad. Sci 2007, 1098, 15–21. - PubMed
-
- Thomas E; Hay EM; Hajeer A; Silman AJ Sjogren’s syndrome: a community-based study of prevalence and impact. Br J. Rheumatol 1998, 37 (10), 1069–76. - PubMed
-
- Bloch KJ; Buchanan WW; Wohl MJ; Bunim JJ Sjoegren’s Syndrome. A Clinical, Pathological, and Serological Study of Sixty-Two Cases. Medicine (Philadelphia, PA, U. S.) 1965, 44, 187–231. - PubMed
-
- Daniels TE; Criswell LA; Shiboski C; Shiboski S; Lanfranchi H; Dong Y; Schiodt M; Umehara H; Sugai S; Challacombe S; Greenspan JS Sjogren’s International Collaborative Clinical Alliance Research, G., An early view of the international Sjogren’s syndrome registry. Arthritis Rheum. 2009, 61 (5), 711–4. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

