Murine models of scrub typhus associated with host control of Orientia tsutsugamushi infection
- PMID: 28282373
- PMCID: PMC5362142
- DOI: 10.1371/journal.pntd.0005453
Murine models of scrub typhus associated with host control of Orientia tsutsugamushi infection
Abstract
Background: Scrub typhus, a febrile illness of substantial incidence and mortality, is caused by infection with the obligately intracellular bacterium Orientia tsutsugamushi. It is estimated that there are more than one million cases annually transmitted by the parasitic larval stage of trombiculid mites in the Asia-Pacific region. The antigenic and genetic diversity of the multiple strains of O. tsutsugamushi hinders the advancement of laboratory diagnosis, development of long-lasting vaccine-induced protection, and interpretation of clinical infection. Despite the life-threatening severity of the illness in hundreds of thousands of cases annually, 85-93% of patients survive, often without anti-rickettsial treatment. To more completely understand the disease caused by Orientia infection, animal models which closely correlate with the clinical manifestations, target cells, organ involvement, and histopathologic lesions of human cases of scrub typhus should be employed. Previously, our laboratory has extensively characterized two relevant C57BL/6 mouse models using O. tsutsugamushi Karp strain: a route-specific intradermal model of infection and persistence and a hematogenously disseminated dose-dependent lethal model.
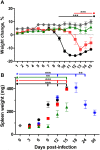
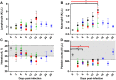
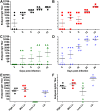
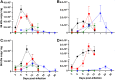
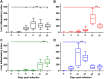
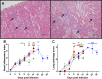
Principal findings: To complement the lethal model, here we illustrate a sublethal model in the same mouse strain using the O. tsutsugamushi Gilliam strain, which resulted in dose-dependent severity of illness, weight loss, and systemic dissemination to endothelial cells of the microcirculation and mononuclear phagocytic cells. Histopathologic lesions included expansion of the pulmonary interstitium by inflammatory cell infiltrates and multifocal hepatic lesions with mononuclear cellular infiltrates, renal interstitial lymphohistiocytic inflammation, mild meningoencephalitis, and characteristic typhus nodules.
Significance: These models parallel characteristics of human cases of scrub typhus, and will be used in concert to understand differences in severity which lead to lethality or host control of the infection and to address the explanation for short duration of heterologous immunity in Orientia infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus [corrected].PLoS Negl Trop Dis. 2014 Jul 10;8(7):e2966. doi: 10.1371/journal.pntd.0002966. eCollection 2014 Jul. PLoS Negl Trop Dis. 2014. PMID: 25010338 Free PMC article.
-
A time-course comparative clinical and immune response evaluation study between the human pathogenic Orientia tsutsugamushi strains: Karp and Gilliam in a rhesus macaque (Macaca mulatta) model.PLoS Negl Trop Dis. 2022 Aug 4;16(8):e0010611. doi: 10.1371/journal.pntd.0010611. eCollection 2022 Aug. PLoS Negl Trop Dis. 2022. PMID: 35925895 Free PMC article.
-
An intradermal inoculation model of scrub typhus in Swiss CD-1 mice demonstrates more rapid dissemination of virulent strains of Orientia tsutsugamushi.PLoS One. 2013;8(1):e54570. doi: 10.1371/journal.pone.0054570. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342173 Free PMC article.
-
Approaches to vaccines against Orientia tsutsugamushi.Front Cell Infect Microbiol. 2013 Jan 4;2:170. doi: 10.3389/fcimb.2012.00170. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316486 Free PMC article. Review.
-
An Update on Host-Pathogen Interplay and Modulation of Immune Responses during Orientia tsutsugamushi Infection.Clin Microbiol Rev. 2018 Jan 31;31(2):e00076-17. doi: 10.1128/CMR.00076-17. Print 2018 Apr. Clin Microbiol Rev. 2018. PMID: 29386235 Free PMC article. Review.
Cited by
-
Polarized lung inflammation and Tie2/angiopoietin-mediated endothelial dysfunction during severe Orientia tsutsugamushi infection.PLoS Negl Trop Dis. 2020 Mar 2;14(3):e0007675. doi: 10.1371/journal.pntd.0007675. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32119672 Free PMC article.
-
Stomach as the target organ of Rickettsia heilongjiangensis infection in C57BL/6 mice identified by click chemistry.Commun Biol. 2024 Jun 29;7(1):784. doi: 10.1038/s42003-024-06468-z. Commun Biol. 2024. PMID: 38951577 Free PMC article.
-
Neuroinflammation associated with scrub typhus and spotted fever group rickettsioses.PLoS Negl Trop Dis. 2020 Oct 22;14(10):e0008675. doi: 10.1371/journal.pntd.0008675. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33091013 Free PMC article. Review.
-
Comparison of Lethal and Nonlethal Mouse Models of Orientia tsutsugamushi Infection Reveals T-Cell Population-Associated Cytokine Signatures Correlated with Lethality and Protection.Trop Med Infect Dis. 2021 Jul 2;6(3):121. doi: 10.3390/tropicalmed6030121. Trop Med Infect Dis. 2021. PMID: 34287349 Free PMC article.
-
Pathological Responses in Asian House Shrews (Suncus murinus) to the Naturally Acquired Orientia tsutsugamushi Infection.Microorganisms. 2024 Apr 7;12(4):748. doi: 10.3390/microorganisms12040748. Microorganisms. 2024. PMID: 38674692 Free PMC article.
References
-
- Ghorbani RP, Ghorbani AJ, Jain MK, Walker DH (1997) A case of scrub typhus probably acquired in Africa. Clinical infectious diseases 25: 1473–1474. - PubMed
-
- Giroud P, Jadin J (1951) [The prevalence of Rickettsia orientalis antibodies among natives and asiatics living in Ruanda-Urundi. (Belgian Congo)]. Bulletin de la Societe de pathologie exotique et de ses filiales 44: 50–51. - PubMed
-
- Osuga K, Kimura M, Goto H, Shimada K, Suto T (1991) A case of tsutsugamushi disease probably contracted in Africa. Eur J Clin Microbiol Infect Dis 10: 95–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

