Zebrafish Embryo as an In Vivo Model for Behavioral and Pharmacological Characterization of Methylxanthine Drugs
- PMID: 28282918
- PMCID: PMC5372612
- DOI: 10.3390/ijms18030596
Zebrafish Embryo as an In Vivo Model for Behavioral and Pharmacological Characterization of Methylxanthine Drugs
Abstract
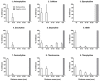
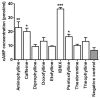
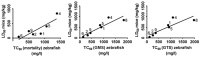
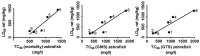
Zebrafish embryo is emerging as an important tool for behavior analysis as well as toxicity testing. In this study, we compared the effect of nine different methylxanthine drugs using zebrafish embryo as a model. We performed behavioral analysis, biochemical assay and Fish Embryo Toxicity (FET) test in zebrafish embryos after treatment with methylxanthines. Each drug appeared to behave in different ways and showed a distinct pattern of results. Embryos treated with seven out of nine methylxanthines exhibited epileptic-like pattern of movements, the severity of which varied with drugs and doses used. Cyclic AMP measurement showed that, despite of a significant increase in cAMP with some compounds, it was unrelated to the observed movement behavior changes. FET test showed a different pattern of toxicity with different methylxanthines. Each drug could be distinguished from the other based on its effect on mortality, morphological defects and teratogenic effects. In addition, there was a strong positive correlation between the toxic doses (TC50) calculated in zebrafish embryos and lethal doses (LD50) in rodents obtained from TOXNET database. Taken together, all these findings elucidate the potentiality of zebrafish embryos as an in vivo model for behavioral and toxicity testing of methylxanthines and other related compounds.
Keywords: Fish Embryo Toxicity test; behavior; cyclic AMP; methylxanthine; movement; zebrafish embryos.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


References
-
- Talik P., Krzek J., Ekiert R.J. Analytical techniques used for determination of methylxanthines and their analogues—Recent advances. Sep. Purif. Rev. 2012;41:1–61. doi: 10.1080/15422119.2011.569047. - DOI
-
- US Department of Agriculture Economic Research Service. Food Contributions of Nonalcoholic Beverages to the U.S. Diet. [(accessed on 27 February 2017)]; Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=44713.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

