Effects of Resveratrol Supplementation on Methotrexate Chemotherapy-Induced Bone Loss
- PMID: 28282956
- PMCID: PMC5372918
- DOI: 10.3390/nu9030255
Effects of Resveratrol Supplementation on Methotrexate Chemotherapy-Induced Bone Loss
Abstract
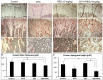
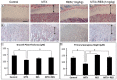
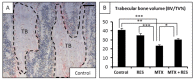
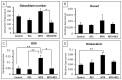
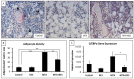
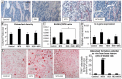
Intensive cancer chemotherapy is known to cause bone defects, which currently lack treatments. This study investigated the effects of polyphenol resveratrol (RES) in preventing bone defects in rats caused by methotrexate (MTX), a commonly used antimetabolite in childhood oncology. Young rats received five daily MTX injections at 0.75 mg/kg/day. RES was orally gavaged daily for seven days prior to, and during, five-day MTX administration. MTX reduced growth plate thickness, primary spongiosa height, trabecular bone volume, increased marrow adipocyte density, and increased mRNA expression of the osteogenic, adipogenic, and osteoclastogenic factors in the tibial bone. RES at 10 mg/kg was found not to affect bone health in normal rats, but to aggravate the bone damage in MTX-treated rats. However, RES supplementation at 1 mg/kg preserved the growth plate, primary spongiosa, bone volume, and lowered the adipocyte density. It maintained expression of genes involved in osteogenesis and decreased expression of adipogenic and osteoclastogenic factors. RES suppressed osteoclast formation ex vivo of bone marrow cells from the treated rats. These data suggest that MTX can enhance osteoclast and adipocyte formation and cause bone loss, and that RES supplementation at 1 mg/kg may potentially prevent these bone defects.
Keywords: resveratrol; bone growth arrest; adipocytes; bone loss; bone marrow adiposity; cancer chemotherapy; growth plate; methotrexate; osteoblasts; osteoclasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Corrie P.G. Cytotoxic chemotherapy: Clinical aspects. Medicine. 2008;36:24–28. doi: 10.1016/j.mpmed.2007.10.012. - DOI
-
- Sridhar T., Symonds R.P. Principles of chemotherapy and radiotherapy. Obstet. Gynaecol. Reprod. Med. 2009;19:61–67. doi: 10.1016/j.ogrm.2008.11.011. - DOI
-
- Fan C., Cool J.C., Scherer M.A., Foster B.K., Shandala T., Tapp H., Xian C.J. Damaging effects of chronic low-dose methotrexate usage on primary bone formation in young rats and potential protective effects of folinic acid supplementary treatment. Bone. 2009;44:61–70. doi: 10.1016/j.bone.2008.09.014. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

