RETRACTED: Combined Effect of Silica Nanoparticles and Benzo[a]pyrene on Cell Cycle Arrest Induction and Apoptosis in Human Umbilical Vein Endothelial Cells
- PMID: 28282959
- PMCID: PMC5369125
- DOI: 10.3390/ijerph14030289
RETRACTED: Combined Effect of Silica Nanoparticles and Benzo[a]pyrene on Cell Cycle Arrest Induction and Apoptosis in Human Umbilical Vein Endothelial Cells
Retraction in
-
RETRACTED: Asweto et al. Combined Effect of Silica Nanoparticles and Benzo[a]pyrene on Cell Cycle Arrest Induction and Apoptosis in Human Umbilical Vein Endothelial Cells. Int. J. Environ. Res. Public Health 2017, 14, 289.Int J Environ Res Public Health. 2025 Mar 27;22(4):508. doi: 10.3390/ijerph22040508. Int J Environ Res Public Health. 2025. PMID: 40164541 Free PMC article.
Abstract
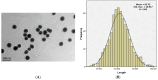
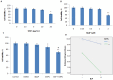
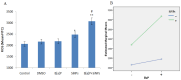
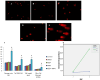
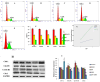
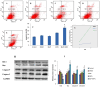
Particulate matter (PM) such as ultrafine particulate matter (UFP) and the organic compound pollutants such as polycyclic aromatic hydrocarbon (PAH) are widespread in the environment. UFP and PAH are present in the air, and their presence may enhance their individual adverse effects on human health. However, the mechanism and effect of their combined interactions on human cells are not well understood. We investigated the combined toxicity of silica nanoparticles (SiNPs) (UFP) and Benzo[a]pyrene (B[a]P) (PAH) on human endothelial cells. Human umbilical vascular endothelial cells (HUVECs) were exposed to SiNPs or B[a]P, or a combination of SiNPs and B[a]P. The toxicity was investigated by assessing cellular oxidative stress, DNA damage, cell cycle arrest, and apoptosis. Our results show that SiNPs were able to induce reactive oxygen species generation (ROS). B[a]P, when acting alone, had no toxicity effect. However, a co-exposure of SiNPs and B[a]P synergistically induced DNA damage, oxidative stress, cell cycle arrest at the G2/M check point, and apoptosis. The co-exposure induced G2/M arrest through the upregulation of Chk1 and downregulation of Cdc25C, cyclin B1. The co-exposure also upregulated bax, caspase-3, and caspase-9, the proapoptic proteins, while down-regulating bcl-2, which is an antiapoptotic protein. These results show that interactions between SiNPs and B[a]P synergistically potentiated toxicological effects on HUVECs. This information should help further our understanding of the combined toxicity of PAH and UFP.
Keywords: Benzo[a]pyrene; HUVECs; co-exposure toxicity; silica nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Raaschou-Nielsen O., Andersen Z.J., Beelen R., Samoli E., Stafoggia M., Weinmayr G., Hoffmann B., Fischer P., Nieuwenhijisen M.J., Brunekreef B., et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. - DOI - PubMed
-
- Shah A.S., Langrish J.P., Nair H., McAllister D.A., Hunter A.L., Donaldson K., Nair H., McAllister D.A., Hunter A.L., Donaldson K., et al. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3. - DOI - PMC - PubMed
-
- Brook R.D., Rajagopalan S., Pope C.A., Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A., et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

