Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: in vitro and in vivo evaluation
- PMID: 28282989
- PMCID: PMC8240974
- DOI: 10.1080/10717544.2017.1284945
Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: in vitro and in vivo evaluation
Abstract
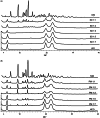
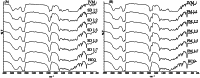
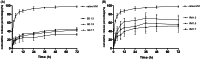
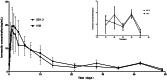
This work aimed to develop a sustained release solid dispersion of ivermectin (IVM-SD) in a lipid matrix (hydrogenated castor oil, HCO) for subcutaneous delivery. Solvent-melting technology was employed to prepare IVM-SDs using HCO. The physicochemical properties of the IVM-SDs were evaluated by scanning electron microscopy (SEM), X-ray powder diffraction (XRPD), and Fourier transform infrared spectroscopy (FTIR). The release of IVM from IVM-SDs was evaluated with HPLC in vitro. Pharmacokinetics of IVM was studied in rabbits following a single subcutaneous administration of IVM-SD formulations. The efficacy of IVM-SD against the ear mange mite was evaluated in rabbits. IVM was completely dispersed in HCO in an amorphous state at a drug:carrier ratio lower than 1:3. No chemical interactions between drug and carrier were found besides hydrogen bonding for the amorphous IVM-SDs. The amorphous IVM-SDs formulations exhibited a sustained release of IVM versus physical mixtures (PMs) of IVM and HCO. The drug release decreased as the drug:carrier ratios decreased, and the release kinetics of IVM were controlled via diffusion. Cytotoxicity of IVM-SD to MDCK cells was lower than native IVM. The IVM plasma concentration of SD1:3 remained above 1 ng/mL for 49 d. Higher AUC, MRT, and Tmax values were obtained at a SD1:3 relative to the IVM group. The IVM-SD improved almost 1.1-fold bioavailability of drug compared with IVM in rabbits. IVM-SD could provide longer persistence against rabbit's ear mites than a commercial IVM injection. This study shows that these solid lipid dispersions are a promising approach for the development of subcutaneous IVM formulations.
Keywords: Ivermectin; bioavailability; physicochemical properties; solid lipid dispersion; subcutaneous delivery; sustained release.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by the “Special Fund for Agro-scientific Research in the Public Interest” (Grant no. 201303037), the “Earmarked Fund for Modern Agro-industry Technology Research System” (CARS-37) funded by the Ministry of Agriculture of China, and “Research and Development of a Long-acting Injectable Suspension of Ivermectin (LA-IVM) to Control Parasites in Ruminant Livestock in South Asia, sub-Saharan Africa and China” (OPP1088557) funded by the Bill & Melinda Gates Foundation.
Figures
References
-
- Bassissi F, Lespine A, Alvinerie M. (2006). Assessment of a liposomal formulation of ivermectin in rabbit after a single subcutaneous administration. Parasitol Res 98:244–9 - PubMed
-
- Bowman DD, Fogelson ML, Carbone LG. (1992). Effect of ivermectin on the control of ear mites (Psoroptes cuniculi) in naturally infested rabbits. Am J Vet Res 53:105–9 - PubMed
-
- Camargo JA, Sapin A, Daloz D, et al. . (2010). Ivermectin-loaded microparticles for parenteral sustained release: in vitro characterization and effect of some formulation variables. J Microencapsul 27:609–17 - PubMed
-
- Chokshi RJ, Zia H, Sandhu HK, et al. . (2007). Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution: pros and cons. Drug Deliv 14:33–45 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
