Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response
- PMID: 28283059
- PMCID: PMC5401687
- DOI: 10.1016/j.cell.2017.02.027
Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response
Abstract
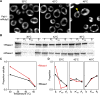
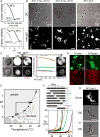
In eukaryotic cells, diverse stresses trigger coalescence of RNA-binding proteins into stress granules. In vitro, stress-granule-associated proteins can demix to form liquids, hydrogels, and other assemblies lacking fixed stoichiometry. Observing these phenomena has generally required conditions far removed from physiological stresses. We show that poly(A)-binding protein (Pab1 in yeast), a defining marker of stress granules, phase separates and forms hydrogels in vitro upon exposure to physiological stress conditions. Other RNA-binding proteins depend upon low-complexity regions (LCRs) or RNA for phase separation, whereas Pab1's LCR is not required for demixing, and RNA inhibits it. Based on unique evolutionary patterns, we create LCR mutations, which systematically tune its biophysical properties and Pab1 phase separation in vitro and in vivo. Mutations that impede phase separation reduce organism fitness during prolonged stress. Poly(A)-binding protein thus acts as a physiological stress sensor, exploiting phase separation to precisely mark stress onset, a broadly generalizable mechanism.
Keywords: RNA-binding protein; energy depletion; heat shock; intrinsically disordered protein; low-complexity region; membraneless organelle; pH; poly(A)-binding protein; quinary structure; stress granules.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Gel or Die: Phase Separation as a Survival Strategy.Cell. 2017 Mar 9;168(6):947-948. doi: 10.1016/j.cell.2017.02.029. Cell. 2017. PMID: 28283065
References
-
- Borzova VA, Markossian KA, Kara DA, Kurganov B. Kinetic regime of dithiothreitol-induced aggregation of bovine serum albumin. Int J Biol Macromol. 2015;80:130–138. - PubMed
-
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. - PubMed
-
- Brangwynne CP, Tompa P, Pappu RV. Polymer physics of intracellular phase transitions. Nat Phys. 2015;11:899–904.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

