Targeting the RhoA-ROCK pathway to reverse T-cell dysfunction in SLE
- PMID: 28283529
- PMCID: PMC5839171
- DOI: 10.1136/annrheumdis-2016-209850
Targeting the RhoA-ROCK pathway to reverse T-cell dysfunction in SLE
Abstract
Objectives: Deregulated production of interleukin (IL)-17 and IL-21 contributes to the pathogenesis of autoimmune disorders such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Production of IL-17 and IL-21 can be regulated by ROCK2, one of the two Rho kinases. Increased ROCK activation was previously observed in an SLE cohort. Here, we evaluated ROCK activity in a new SLE cohort, and an RA cohort, and assessed the ability of distinct inhibitors of the ROCK pathway to suppress production of IL-17 and IL-21 by SLE T cells or human Th17 cells.
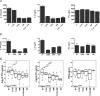
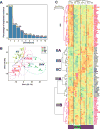
Methods: ROCK activity in peripheral blood mononuclear cells (PBMCs) from 29 patients with SLE, 31 patients with RA and 28 healthy controls was determined by ELISA. SLE T cells or in vitro-differentiated Th17 cells were treated with Y27632 (a pan-ROCK inhibitor), KD025 (a selective ROCK2 inhibitor) or simvastatin (which inhibits RhoA, a major ROCK activator). ROCK activity and IL-17 and IL-21 production were assessed. The transcriptional profile altered by ROCK inhibitors was evaluated by NanoString technology.
Results: ROCK activity levels were significantly higher in patients with SLE and RA than healthy controls. Th17 cells exhibited high ROCK activity that was inhibited by Y27632, KD025 or simvastatin; each also decreased IL-17 and IL-21 production by purified SLE T cells or Th17 cells. Immune profiling revealed both overlapping and distinct effects of the different ROCK inhibitors.
Conclusions: ROCK activity is elevated in PBMCs from patients with SLE and RA. Production of IL-17 and IL-21 by SLE T cells or Th17 cells can furthermore be inhibited by targeting the RhoA-ROCK pathway via both non-selective and selective approaches.
Keywords: Autoimmunity; Systemic Lupus Erythematosus; T Cells.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures
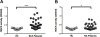

References
-
- Crispin JC, Tsokos GC. Interleukin-17-producing T cells in lupus. Curr Opin Rheumatol. 2010;22(5):499–503. - PubMed
-
- Pernis AB. Th17 cells in rheumatoid arthritis and systemic lupus erythematosus. J Intern Med. 2009;265(6):644–52. - PubMed
-
- Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(2):77–88. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

