Gene Expression Profiling with Cre-Conditional Pseudorabies Virus Reveals a Subset of Midbrain Neurons That Participate in Reward Circuitry
- PMID: 28283558
- PMCID: PMC5391685
- DOI: 10.1523/JNEUROSCI.3193-16.2017
Gene Expression Profiling with Cre-Conditional Pseudorabies Virus Reveals a Subset of Midbrain Neurons That Participate in Reward Circuitry
Abstract
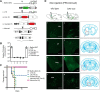
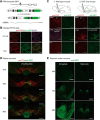
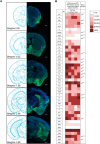
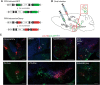
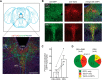
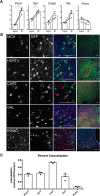
The mesolimbic dopamine pathway receives inputs from numerous regions of the brain as part of a neural system that detects rewarding stimuli and coordinates a behavioral response. The capacity to simultaneously map and molecularly define the components of this complex multisynaptic circuit would thus advance our understanding of the determinants of motivated behavior. To accomplish this, we have constructed pseudorabies virus (PRV) strains in which viral propagation and fluorophore expression are activated only after exposure to Cre recombinase. Once activated in Cre-expressing neurons, the virus serially labels chains of presynaptic neurons. Dual injection of GFP and mCherry tracing viruses simultaneously illuminates nigrostriatal and mesolimbic circuitry and shows no overlap, demonstrating that PRV transmission is confined to synaptically connected neurons. To molecularly profile mesolimbic dopamine neurons and their presynaptic inputs, we injected Cre-conditional GFP virus into the NAc of (anti-GFP) nanobody-L10 transgenic mice and immunoprecipitated translating ribosomes from neurons infected after retrograde tracing. Analysis of purified RNA revealed an enrichment of transcripts expressed in neurons of the dorsal raphe nuclei and lateral hypothalamus that project to the mesolimbic dopamine circuit. These studies identify important inputs to the mesolimbic dopamine pathway and further show that PRV circuit-directed translating ribosome affinity purification can be broadly applied to identify molecularly defined neurons comprising complex, multisynaptic circuits.SIGNIFICANCE STATEMENT The mesolimbic dopamine circuit integrates signals from key brain regions to detect and respond to rewarding stimuli. To further define this complex multisynaptic circuit, we constructed a panel of Cre recombinase-activated pseudorabies viruses (PRVs) that enabled retrograde tracing of neural inputs that terminate on Cre-expressing neurons. Using these viruses and Retro-TRAP (translating ribosome affinity purification), a previously reported molecular profiling method, we developed a novel technique that provides anatomic as well as molecular information about the neural components of polysynaptic circuits. We refer to this new method as PRV-Circuit-TRAP (PRV circuit-directed TRAP). Using it, we have identified major projections to the mesolimbic dopamine circuit from the lateral hypothalamus and dorsal raphe nucleus and defined a discrete subset of transcripts expressed in these projecting neurons, which will allow further characterization of this important pathway. Moreover, the method we report is general and can be applied to the study of other neural circuits.
Keywords: RNASeq; mesolimbic; pseudorabies virus.
Copyright © 2017 the authors 0270-6474/17/374129-17$15.00/0.
Figures

Similar articles
-
A dual infection pseudorabies virus conditional reporter approach to identify projections to collateralized neurons in complex neural circuits.PLoS One. 2011;6(6):e21141. doi: 10.1371/journal.pone.0021141. Epub 2011 Jun 16. PLoS One. 2011. PMID: 21698154 Free PMC article.
-
Development of Cre-dependent retrograde trans-multisynaptic tracer based on pseudorabies virus bartha strain.Mol Brain. 2025 Apr 14;18(1):33. doi: 10.1186/s13041-025-01204-y. Mol Brain. 2025. PMID: 40229811 Free PMC article.
-
Molecular characterization of neuronal cell types based on patterns of projection with Retro-TRAP.Nat Protoc. 2015 Sep;10(9):1319-27. doi: 10.1038/nprot.2015.087. Epub 2015 Aug 6. Nat Protoc. 2015. PMID: 26247298
-
Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations.J Neurosci Methods. 2000 Nov 15;103(1):51-61. doi: 10.1016/s0165-0270(00)00295-8. J Neurosci Methods. 2000. PMID: 11074095 Review.
-
Exploiting circuit-specific spread of pseudorabies virus in the central nervous system: insights to pathogenesis and circuit tracers.J Infect Dis. 2002 Dec 1;186 Suppl 2:S209-14. doi: 10.1086/344278. J Infect Dis. 2002. PMID: 12424699 Review.
Cited by
-
Unveiling Hypothalamic Molecular Signatures via Retrograde Viral Tracing and Single-Cell Transcriptomics.Sci Data. 2023 Dec 4;10(1):861. doi: 10.1038/s41597-023-02789-6. Sci Data. 2023. PMID: 38049462 Free PMC article.
-
Mapping Connectivity Amongst Interneuronal Components of the Locomotor CPG.Front Cell Neurosci. 2019 Oct 4;13:443. doi: 10.3389/fncel.2019.00443. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31636541 Free PMC article.
-
Multi-Platform Sequencing Approach Reveals a Novel Transcriptome Profile in Pseudorabies Virus.Front Microbiol. 2018 Jan 22;8:2708. doi: 10.3389/fmicb.2017.02708. eCollection 2017. Front Microbiol. 2018. PMID: 29403453 Free PMC article.
-
Differences in neurotropism and neurotoxicity among retrograde viral tracers.Mol Neurodegener. 2019 Feb 8;14(1):8. doi: 10.1186/s13024-019-0308-6. Mol Neurodegener. 2019. PMID: 30736827 Free PMC article.
-
Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse.PLoS One. 2019 Mar 27;14(3):e0213927. doi: 10.1371/journal.pone.0213927. eCollection 2019. PLoS One. 2019. PMID: 30917148 Free PMC article.
References
-
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25:25–29. 10.1038/75556 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
