Effects of a detergent micelle environment on P-glycoprotein (ABCB1)-ligand interactions
- PMID: 28283574
- PMCID: PMC5409473
- DOI: 10.1074/jbc.M116.771634
Effects of a detergent micelle environment on P-glycoprotein (ABCB1)-ligand interactions
Abstract
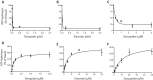
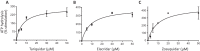
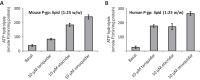
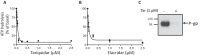
P-glycoprotein (P-gp) is a multidrug transporter that uses energy from ATP hydrolysis to export many structurally dissimilar hydrophobic and amphipathic compounds, including anticancer drugs from cells. Several structural studies on purified P-gp have been reported, but only limited and sometimes conflicting information is available on ligand interactions with the isolated transporter in a dodecyl-maltoside detergent environment. In this report we compared the biochemical properties of P-gp in native membranes, detergent micelles, and when reconstituted in artificial membranes. We found that the modulators zosuquidar, tariquidar, and elacridar stimulated the ATPase activity of purified human or mouse P-gp in a detergent micelle environment. In contrast, these drugs inhibited ATPase activity in native membranes or in proteoliposomes, with IC50 values in the 10-40 nm range. Similarly, a 30-150-fold decrease in the apparent affinity for verapamil and cyclic peptide inhibitor QZ59-SSS was observed in detergent micelles compared with native or artificial membranes. Together, these findings demonstrate that the high-affinity site is inaccessible because of either a conformational change or binding of detergent at the binding site in a detergent micelle environment. The ligands bind to a low-affinity site, resulting in altered modulation of P-gp ATPase activity. We, therefore, recommend studying structural and functional aspects of ligand interactions with purified P-gp and other ATP-binding cassette transporters that transport amphipathic or hydrophobic substrates in a detergent-free native or artificial membrane environment.
Keywords: ABC transporter; ATP hydrolysis; P-glycoprotein; detergent micelles; docking; drug resistance; molecular modeling; multidrug transporter; nanodiscs; proteoliposomes.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- Ambudkar S. V., Kimchi-Sarfaty C., Sauna Z. E., and Gottesman M. M. (2003) P-glycoprotein: from genomics to mechanism. Oncogene 22, 7468–7485 - PubMed
-
- Ambudkar S. V., Dey S., Hrycyna C. A., Ramachandra M., Pastan I., and Gottesman M. M. (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 39, 361–398 - PubMed
-
- Gottesman M. M., and Pastan I. (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62, 385–427 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

