Amyotrophic lateral sclerosis, gene deregulation in the anterior horn of the spinal cord and frontal cortex area 8: implications in frontotemporal lobar degeneration
- PMID: 28283675
- PMCID: PMC5391234
- DOI: 10.18632/aging.101195
Amyotrophic lateral sclerosis, gene deregulation in the anterior horn of the spinal cord and frontal cortex area 8: implications in frontotemporal lobar degeneration
Abstract
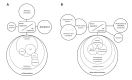
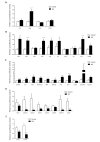
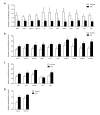
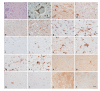
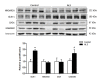
Transcriptome arrays identifies 747 genes differentially expressed in the anterior horn of the spinal cord and 2,300 genes differentially expressed in frontal cortex area 8 in a single group of typical sALS cases without frontotemporal dementia compared with age-matched controls. Main up-regulated clusters in the anterior horn are related to inflammation and apoptosis; down-regulated clusters are linked to axoneme structures and protein synthesis. In contrast, up-regulated gene clusters in frontal cortex area 8 involve neurotransmission, synaptic proteins and vesicle trafficking, whereas main down-regulated genes cluster into oligodendrocyte function and myelin-related proteins. RT-qPCR validates the expression of 58 of 66 assessed genes from different clusters. The present results: a. reveal regional differences in de-regulated gene expression between the anterior horn of the spinal cord and frontal cortex area 8 in the same individuals suffering from sALS; b. validate and extend our knowledge about the complexity of the inflammatory response in the anterior horn of the spinal cord; and c. identify for the first time extensive gene up-regulation of neurotransmission and synaptic-related genes, together with significant down-regulation of oligodendrocyte- and myelin-related genes, as important contributors to the pathogenesis of frontal cortex alterations in the sALS/frontotemporal lobar degeneration spectrum complex at stages with no apparent cognitive impairment.
Keywords: amyotrophic lateral sclerosis; excitotoxicity; frontal cortex; frontotemporal lobar degeneration; neuroinflammation; spinal cord.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Horobágyi T, Cairns NJ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration. In: Kovacs GG, editor. Neuropathology of neurodegenerative diseases: a practical guide. Cambridge Press; Cambridge: 2015. pp. 209–248.
-
- Strong MJ, Hortobágyi T, Okamoto K, Kato S. Amyotrophic lateral sclerosis, primary lateral sclerosis, and spinal muscular atrophy. In: Dickson DW, editor. Neurodegeneration: the molecular pathology of dementia and movement disorders. 2nd. Wiley-Blackwell; Oxford: 2011. pp. 418–433.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

