Magnetic resonance diffusion tensor imaging for the pedunculopontine nucleus: proof of concept and histological correlation
- PMID: 28283747
- PMCID: PMC5610900
- DOI: 10.1007/s00429-016-1356-0
Magnetic resonance diffusion tensor imaging for the pedunculopontine nucleus: proof of concept and histological correlation
Abstract
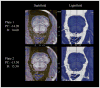
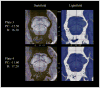
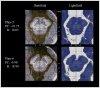
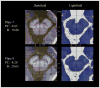
The pedunculopontine nucleus (PPN) has been proposed as target for deep brain stimulation (DBS) in patients with postural instability and gait disorders due to its involvement in muscle tonus adjustments and control of locomotion. However, it is a deep-seated brainstem nucleus without clear imaging or electrophysiological markers. Some studies suggested that diffusion tensor imaging (DTI) may help guiding electrode placement in the PPN by showing the surrounding fiber bundles, but none have provided a direct histological correlation. We investigated DTI fractional anisotropy (FA) maps from in vivo and in situ post-mortem magnetic resonance images (MRI) compared to histological evaluations for improving PPN targeting in humans. A post-mortem brain was scanned in a clinical 3T MR system in situ. Thereafter, the brain was processed with a special method ideally suited for cytoarchitectonic analyses. Also, nine volunteers had in vivo brain scanning using the same MRI protocol. Images from volunteers were compared to those obtained in the post-mortem study. FA values of the volunteers were obtained from PPN, inferior colliculus, cerebellar crossing fibers and medial lemniscus using histological data and atlas information. FA values in the PPN were significantly lower than in the surrounding white matter region and higher than in areas with predominantly gray matter. In Nissl-stained histologic sections, the PPN extended for more than 10 mm in the rostro-caudal axis being closely attached to the lateral parabrachial nucleus. Our DTI analyses and the spatial correlation with histological findings proposed a location for PPN that matched the position assigned to this nucleus in the literature. Coregistration of neuroimaging and cytoarchitectonic features can add value to help establishing functional architectonics of the PPN and facilitate neurosurgical targeting of this extended nucleus.
Keywords: Deep brain stimulation; Diffusion tensor imaging; Histology; Pedunculopontine nucleus.
Conflict of interest statement
Figures


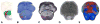

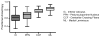

References
-
- Afshar F, Watkins ES, Yap JC. Stereotaxic atlas of the human brainstem and cerebellar nuclei: a variability study. Raven Press; New York: 1978.
-
- Alegro M, Alho EJL, Lopes R, Zollei L, Amaro-Junior E. A Computational Pipeline for Full Brain Histology to MRI registration. Organization for Human Brain Mapping; Honolulu, Hawaii, USA: 2015.
-
- Avants BB, Tustison N, Song G. Advanced normalization tools (stnava.github.io/ants) USA: 2015.
-
- Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. 2013;80(12):1148–1155. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

