Metabolic carbonyl reduction of anthracyclines - role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents
- PMID: 28283780
- PMCID: PMC5418329
- DOI: 10.1007/s10637-017-0443-2
Metabolic carbonyl reduction of anthracyclines - role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents
Abstract
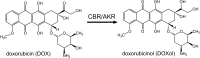
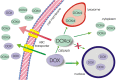
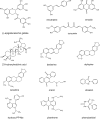
Anthracycline antibiotics (ANT), such as doxorubicin or daunorubicin, are a class of anticancer drugs that are widely used in oncology. Although highly effective in cancer therapy, their usefulness is greatly limited by their cardiotoxicity. Possible mechanisms of ANT cardiotoxicity include their conversion to secondary alcohol metabolites (i.e. doxorubicinol, daunorubicinol) catalyzed by carbonyl reductases (CBR) and aldo-keto reductases (AKR). These metabolites are suspected to be more cardiotoxic than their parent compounds. Moreover, overexpression of ANT-reducing enzymes (CBR and AKR) are found in many ANT-resistant cancers. The secondary metabolites show decreased cytotoxic properties and are more susceptible to ABC-mediated efflux than their parent compounds; thus, metabolite formation is considered one of the mechanisms of cancer resistance. Inhibitors of CBR and AKR were found to reduce the cardiotoxicity of ANT and the resistance of cancer cells, and therefore are being investigated as prospective cardioprotective and chemosensitizing drug candidates. In this review, the significance of a two-electron reduction of ANT, including daunorubicin, epirubicin, idarubicin, valrubicin, amrubicin, aclarubicin, and especially doxorubicin, is described with respect to toxicity and efficacy of therapy. Additionally, CBR and AKR inhibitors, including monoHER, curcumin, (-)-epigallocatechin gallate, resveratrol, berberine or pixantrone, and their modulating effect on the activity of ANT is characterized and discussed as potential mechanism of action for novel therapeutics in cancer treatment.
Keywords: Anthracyclines; Anticancer agents; Cardiotoxicity; Drug metabolism; Pharmacokinetics; Resistance.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
The study was supported by Jagiellonian University Medical College (K/ZDS/005488).
Ethical Approval
This article does not contain a studies with human participants or animals.
Figures

References
-
- Karch A, Koch A, Grünwald V. A phase II trial comparing pazopanib with doxorubicin as first-line treatment in elderly patients with metastatic or advanced soft tissue sarcoma (EPAZ): study protocol for a randomized controlled trial. Trials. 2016;17(1):312. doi: 10.1186/s13063-016-1434-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

