Nicotine Population Pharmacokinetics in Healthy Adult Smokers: A Retrospective Analysis
- PMID: 28283988
- PMCID: PMC5681983
- DOI: 10.1007/s13318-017-0405-2
Nicotine Population Pharmacokinetics in Healthy Adult Smokers: A Retrospective Analysis
Abstract
Background and objective: Characterizing nicotine pharmacokinetics is challenging in the presence of background exposure. We performed a combined retrospective population pharmacokinetic analysis of 8 trials, including exposure to Tobacco Heating System and cigarettes (both inhaled), nicotine nasal spray and oral nicotine gum.
Method: Data from 4 single product use trials were used to develop a population pharmacokinetic model with Phoenix® NLME™ and to derive exposure parameters. Data from 4 separate ad libitum use studies were used for external validation. A total of 702 healthy adult smokers (54% males; 21-66 years of age; smoking ≥10 cigarettes/day; from US, Europe and Japan) were eligible for participation.
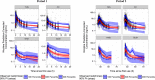
Results: Two-compartment linear disposition combined with zero-order absorption model was adequate to describe nicotine pharmacokinetics, and a mono-exponentially decreasing background component was utilized to account for nicotine carry-over effects. Apparent nicotine clearance was typically 0.407 L/min in males and 26% higher in females (68% inter-individual variability). Bioavailability was product-specific, decreased with increasing nicotine ISO yield, and increased with increasing body weight. Absorption duration was apparently prolonged with nicotine gum. The typical initial and terminal half-lives were 1.35 and 17 h, respectively. The presence of menthol did not impact the determinants of the area under the curve. The model adequately described the external validation data.
Conclusions: The population model was able to describe in different populations the nicotine pharmacokinetics after single product use and after 4 days of ad libitum use of Tobacco Heating System, cigarettes, and of different nicotine replacement therapies with various routes of administration.
Conflict of interest statement
Funding
Certara received funds from Philip Morris International for designing and conducting the analysis, and for writing the manuscript.
Conflict of interest
Author P. B. is a former employee of Philip Morris International. Authors N. L., R. W., and F. L. are employees of Philip Morris Product S.A.. Authors M. M. and H. M. are employees of Certara.
Ethical approval
The above mentioned model was built using the datasets of 8 clinical studies. All procedures performed in these studies involving human participants were conducted in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from each subject who participated in one of the eight trials prior to performing any study-specific procedures.
Figures

References
-
- U.S Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2004.
-
- U.S Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. 2014.
-
- Bilano V, Gilmour S, Moffiet T, d’Espaignet ET, Stevens GA, Commar A, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385(9972):966–976. doi: 10.1016/S0140-6736(15)60264-1. - DOI - PubMed
-
- Roethig HJ, Koval T, Muhammad-Kah R, Jin Y, Mendes P, Unverdorben M. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regul Toxicol Pharmacol. 2010;57(2–3):333–337. doi: 10.1016/j.yrtph.2010.04.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

