Cerenkov luminescence imaging: physics principles and potential applications in biomedical sciences
- PMID: 28283990
- PMCID: PMC5346099
- DOI: 10.1186/s40658-017-0181-8
Cerenkov luminescence imaging: physics principles and potential applications in biomedical sciences
Abstract
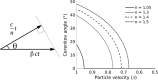
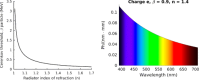
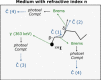
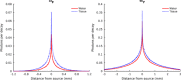
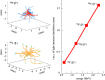
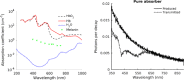
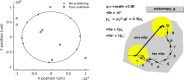
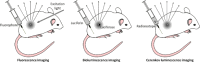
Cerenkov luminescence imaging (CLI) is a novel imaging modality to study charged particles with optical methods by detecting the Cerenkov luminescence produced in tissue. This paper first describes the physical processes that govern the production and transport in tissue of Cerenkov luminescence. The detectors used for CLI and their most relevant specifications to optimize the acquisition of the Cerenkov signal are then presented, and CLI is compared with the other optical imaging modalities sharing the same data acquisition and processing methods. Finally, the scientific work related to CLI and the applications for which CLI has been proposed are reviewed. The paper ends with some considerations about further perspectives for this novel imaging modality.
Keywords: Cerenkov luminescence imaging; Nuclear medicine; Optical imaging.
Figures

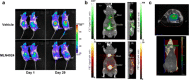
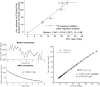
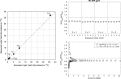
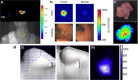
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

