Novel interferon-gamma assays for diagnosing tuberculosis in young children in India
- PMID: 28284256
- PMCID: PMC5453635
- DOI: 10.5588/ijtld.16.0428
Novel interferon-gamma assays for diagnosing tuberculosis in young children in India
Abstract
Setting: The tuberculin skin test (TST) and interferon-gamma release assays (IGRAs) are used as supportive evidence to diagnose active tuberculosis (TB). Novel IGRAs could improve diagnosis, but data are lacking in young children.
Design: Children (age 5 years) with suspected TB were prospectively screened at a tertiary hospital in Pune, India; the children underwent TST, and standard (early secretory antigenic target 6 and culture filtrate protein 10) and enhanced (five additional novel antigens) enzyme-linked immunospot (ELISpot) assays.
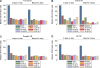
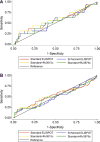
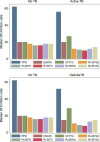
Results: Of 313 children (median age 30 months) enrolled, 92% had received bacille Calmette-Guérin vaccination, 53% were malnourished and 9% were coinfected with the human immunodeficiency virus (HIV); 48 (15%) had TB, 128 (41%) did not, and TB could not be ruled out in 137 (44%). The sensitivity of enhanced (45%) and standard (42%) ELISpot assays for diagnosing TB was better than that of TST (20%) (P 0.03); however, enhanced ELISpot was not more sensitive than the standard ELISpot assay (P = 0.50). The specificity of enhanced ELISpot, standard ELISpot and TST was respectively 82% (95%CI 74-89), 88% (95%CI 81-94) and 98% (95%CI 93-100). Rv3879c and Rv3615c, previously reported to be promising antigens, failed to improve the diagnostic performance of the ELISpot assay.
Conclusion: The TST and the standard and novel ELISpot assays performed poorly in diagnosing active TB among young children in India.
Conflict of interest statement
Conflicts of interest: AL holds several patents in the field of T cell-based diagnosis, some of which are licensed to Oxford Immunotec Ltd, Abingdon, UK. The interferon-gamma ELISpot assay developed by AL for diagnosis of tuberculous infection was commercialised by an Oxford University (Oxford, UK) spin-out company (T-SPOT®.
Figures


Comment in
-
IGRAs for children: the long and winding road.Int J Tuberc Lung Dis. 2017 Apr 1;21(4):364. doi: 10.5588/ijtld.17.0106. Int J Tuberc Lung Dis. 2017. PMID: 28284248 No abstract available.
References
-
- Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Global Health. 2014;2:e453–e459. - PubMed
-
- Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–114. - PubMed
-
- World Health Organization. Global tuberculosis report, 2015. Geneva, Switzerland: WHO; 2015. (WHO/HTM/TB/2015.22).
-
- Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–263. - PubMed
-
- Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Suppl 3):S184–S194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

