Efficacy of Intravenous Iron Sucrose in Hemodialysis Patients with Restless Legs Syndrome (RLS): A Randomized, Placebo-Controlled Study
- PMID: 28285317
- PMCID: PMC5360424
- DOI: 10.12659/msm.900520
Efficacy of Intravenous Iron Sucrose in Hemodialysis Patients with Restless Legs Syndrome (RLS): A Randomized, Placebo-Controlled Study
Abstract
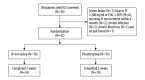
BACKGROUND Restless legs syndrome (RLS) is a common disorder in hemodialysis (HD) patients that causes sleep disturbances and diminished quality of life. Because iron deficiency has been implicated in the pathogenesis of RLS, we sought to investigate the effects of intravenous (IV) iron sucrose on symptoms of RLS in HD patients. MATERIAL AND METHODS The study was a randomized, placebo-controlled study of 1000 mg iron sucrose versus normal saline as placebo. Patients were evaluated at baseline and 2 weeks after the last injection. The severity of RLS was assessed using the International RLS Study Group rating scale (IRLS). Blood samples were taken to measure iron parameters reflecting the iron status, including serum ferritin (SF) concentration, percentage transferrin saturation (TSAT%) and hemoglobin (Hb), and other biochemical parameters as safety assessments, including creatinine (Cr), urea, intact parathyroid hormone (iPTH), and the index of urea clearance (Kt/V). Adverse events were monitored in all subjects during the period of infusion. RESULTS After 2 weeks, IRLS scores decreased more in the IV-iron group (-7.38±2.03) than in the placebo group (-0.81±2.61) (P=0.000). Serum ferritin, TSAT, and hemoglobin increased more in the IV-iron group (227.63±77.64 µg/L; 26.06±7.77%; 13.98±3.62g/L, respectively) than in the placebo group (SF, p=0.000; TSAT, p=0.000; Hb, p=0.000, respectively). There were no significant differences between IV-iron and placebo groups in Cr, urea, iPTH, and Kt/V. No adverse effects were observed in the study. CONCLUSIONS IV iron sucrose is a safe and effective treatment for reducing RLS symptoms in HD patients over the short-term.
References
-
- Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. J Neurosci Res. 2000;62:623–28. - PubMed
-
- Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26:925–35. - PubMed
-
- Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. - PubMed
-
- Merlino G, Lorenzut S, Gigli GL, et al. A case-control study on restless legs syndrome in nondialyzed patients with chronic renal failure. Mov Disord. 2010;25:1019–25. - PubMed
-
- Araujo SM, de Bruin VM, Nepomuceno LA, et al. Restless legs syndrome in end-stage renal disease: Clinical characteristics and associated comorbidities. Sleep Med. 2010;11:785–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical