Canagliflozin as an Initial Therapy in Drug-Naïve Subjects with Type 2 Diabetes Mellitus: A Potential Involvement of Atherogenic Lipids in its Glycemic Efficacy
- PMID: 28285448
- PMCID: PMC5427050
- DOI: 10.1007/s40268-017-0179-7
Canagliflozin as an Initial Therapy in Drug-Naïve Subjects with Type 2 Diabetes Mellitus: A Potential Involvement of Atherogenic Lipids in its Glycemic Efficacy
Abstract
Background and objectives: The aim of this study is to investigate canagliflozin as an initial therapy in type 2 diabetes mellitus and to explore the effects on metabolic parameters in relation to effects on glycemic control.
Subjects and methods: Treatment-naïve subjects with type 2 diabetes mellitus received canagliflozin 50-100 mg/day monotherapy. At 3 months, levels of glycemic and non-glycemic parameters were compared with those at baseline (n = 39). As a comparator, our previous data of baseline glycosylated hemoglobin (HbA1c)-matched treatment-naïve subjects with ipragliflozin 25-50 mg monotherapy (n = 27) were employed.
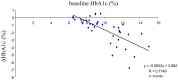
Results: Significant reductions in HbA1c (from 9.96 to 8.33%), fasting blood glucose (-23.9%), homeostasis model assessment-R (HOMA-R, -33.5%), body mass index (-1.8%), and uric acid (UA, -5.2%) levels and significant increases in homeostasis model assessment-B (HOMA-B, 30.1%) levels were observed. Approximately one third of the subjects experienced certain adverse events. Similar results were obtained with ipragliflozin. Baseline levels of HbA1c, triglycerides, non-high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) were chosen as significant contributing factors for the changes in HbA1c levels with canagliflzoin, while only baseline HbA1c levels were selected as such a factor with ipragliflozin. Significant positive correlations between the changes in HbA1c and changes in non-HDL-C (R = 0.3954) or between changes in HbA1c and changes in LDL-C (R = 0.4317) were observed with canagliflozin. With ipragliflozin, no such correlations were noted. No correlations between the changes in HbA1c and changes in body mass index were seen with both drugs.
Conclusions: These results suggest that (1) canagliflozin appears to offer clinically beneficial outcomes as an initial therapy in subjects with type 2 diabetes mellitus, although with certain adverse events. (2) Atherogenic cholesterols including non-HDL-C and LDL-C could be involved in the glycemic efficacy of canagliflozin. This was not the case with ipragliflozin. (3) Unexpectedly, weight reductions with canagliflozin are not associated with its glycemic efficacy.
Conflict of interest statement
Funding
No funding was received for the preparation and conduct of this study.
Conflict of interest
Eiji Kutoh, Asuka Wada, Teruma Murayama, and Yui Takizawa have no conflicts of interest directly relevant to the content of this study.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines.
Consent to participate
All subjects provided informed consent.
Figures
Similar articles
-
Distinct Glucose-Lowering Mechanisms of Ipragliflozin Depending on Body Weight Changes.Drugs R D. 2016 Dec;16(4):369-376. doi: 10.1007/s40268-016-0149-5. Drugs R D. 2016. PMID: 27798769 Free PMC article.
-
Ipragliflozin as an Initial Therapy in Drug Naïve Subjects with Type 2 Diabetes.Drug Res (Stuttg). 2016 Jul;66(7):345-50. doi: 10.1055/s-0035-1569454. Epub 2016 Apr 7. Drug Res (Stuttg). 2016. PMID: 27056638 Clinical Trial.
-
Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study.Curr Med Res Opin. 2014 Feb;30(2):163-75. doi: 10.1185/03007995.2013.850066. Epub 2013 Oct 28. Curr Med Res Opin. 2014. PMID: 24073995 Clinical Trial.
-
A review of the efficacy and safety of canagliflozin in elderly patients with type 2 diabetes.Consult Pharm. 2014;29(5):335-46. doi: 10.4140/TCP.n.2014.335. Consult Pharm. 2014. PMID: 24849690 Review.
-
Canagliflozin: a review of its use in patients with type 2 diabetes mellitus.Drugs. 2014 May;74(7):807-24. doi: 10.1007/s40265-014-0225-5. Drugs. 2014. PMID: 24831734 Review.
Cited by
-
Real-world evidence for long-term safety and effectiveness of ipragliflozin in treatment-naïve versus non-naïve Japanese patients with type 2 diabetes mellitus: subgroup analysis of a 3-year post-marketing surveillance study (STELLA-LONG TERM).Diabetol Int. 2021 Mar 24;12(4):430-444. doi: 10.1007/s13340-021-00501-w. eCollection 2021 Oct. Diabetol Int. 2021. PMID: 34567926 Free PMC article.
-
Two Glucose-Lowering Mechanisms of Canagliflozin Depending on Body Weight Changes in Drug-Naïve Subjects with Type 2 Diabetes.Drugs R D. 2018 Dec;18(4):309-315. doi: 10.1007/s40268-018-0250-z. Drugs R D. 2018. PMID: 30324549 Free PMC article. Clinical Trial.
-
Rationale for the Early Use of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes.Adv Ther. 2019 Oct;36(10):2567-2586. doi: 10.1007/s12325-019-01054-w. Epub 2019 Aug 23. Adv Ther. 2019. PMID: 31444707 Free PMC article. Review.
-
Characterization of Metabolic Parameters in Responders and Nonresponders Treated with Canagliflozin Monotherapy in Drug-naive Subjects with Type 2 Diabetes.Indian J Endocrinol Metab. 2018 Mar-Apr;22(2):185-190. doi: 10.4103/ijem.IJEM_578_17. Indian J Endocrinol Metab. 2018. PMID: 29911028 Free PMC article.
-
Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice.Cardiovasc Diabetol. 2018 Jul 26;17(1):106. doi: 10.1186/s12933-018-0749-1. Cardiovasc Diabetol. 2018. PMID: 30049285 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous