Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis
- PMID: 28285495
- PMCID: PMC6464698
- DOI: 10.1002/14651858.CD011644.pub2
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis
Update in
-
Pharmacological interventions for acute hepatitis C infection.Cochrane Database Syst Rev. 2018 Dec 3;12(12):CD011644. doi: 10.1002/14651858.CD011644.pub3. Cochrane Database Syst Rev. 2018. PMID: 30521693 Free PMC article.
Abstract
Background: Hepatitis C virus (HCV) is a single-stranded RNA (ribonucleic acid) virus that has the potential to cause inflammation of the liver. The traditional definition of acute HCV infection is the first six months following infection with the virus. Another commonly used definition of acute HCV infection is the absence of HCV antibody and subsequent seroconversion (presence of HCV antibody in a person who was previously negative for HCV antibody). Approximately 40% to 95% of people with acute HCV infection develop chronic HCV infection, that is, have persistent HCV RNA in their blood. In 2010, an estimated 160 million people worldwide (2% to 3% of the world's population) had chronic HCV infection. The optimal pharmacological treatment of acute HCV remains controversial. Chronic HCV infection can damage the liver.
Objectives: To assess the comparative benefits and harms of different pharmacological interventions in the treatment of acute HCV infection through a network meta-analysis and to generate rankings of the available pharmacological treatments according to their safety and efficacy. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta-analysis, and instead, we assessed the comparative benefits and harms of different interventions versus each other or versus no intervention using standard Cochrane methodology.
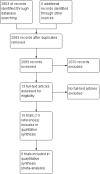
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and randomised controlled trials registers to April 2016 to identify randomised clinical trials on pharmacological interventions for acute HCV infection.
Selection criteria: We included only randomised clinical trials (irrespective of language, blinding, or publication status) in participants with acute HCV infection. We excluded trials which included previously liver transplanted participants and those with other coexisting viral diseases. We considered any of the various pharmacological interventions compared with placebo or each other.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. We calculated the odds ratio (OR) and rate ratio with 95% confidence intervals (CI) using both fixed-effect and random-effects models based on the available-participant analysis with Review Manager 5. We assessed risk of bias according to Cochrane, controlled risk of random errors with Trial Sequential Analysis, and assessed the quality of the evidence using GRADE.
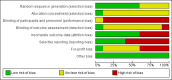
Main results: We identified 10 randomised clinical trials with 488 randomised participants that met our inclusion criteria. All the trials were at high risk of bias in one or more domains. Overall, the evidence for all the outcomes was very low quality evidence. Nine trials (467 participants) provided information for one or more outcomes. Three trials (99 participants) compared interferon-alpha versus no intervention. Three trials (90 participants) compared interferon-beta versus no intervention. One trial (21 participants) compared pegylated interferon-alpha versus no intervention, but it did not provide any data for analysis. One trial (41 participants) compared MTH-68/B vaccine versus no intervention. Two trials (237 participants) compared pegylated interferon-alpha versus pegylated interferon-alpha plus ribavirin. None of the trials compared direct-acting antivirals versus placebo or other interventions. The mean or median follow-up period in the trials ranged from six to 36 months.There was no short-term mortality (less than one year) in any group in any trial except for one trial where one participant died in the pegylated interferon-alpha plus ribavirin group (1/95: 1.1%). In the trials that reported follow-up beyond one year, there were no further deaths. The number of serious adverse events was higher with pegylated interferon-alpha plus ribavirin than with pegylated interferon-alpha (rate ratio 2.74, 95% CI 1.40 to 5.33; participants = 237; trials = 2; I2 = 0%). The proportion of people with any adverse events was higher with interferon-alpha and interferon-beta compared with no intervention (OR 203.00, 95% CI 9.01 to 4574.81; participants = 33; trials = 1 and OR 27.88, 95% CI 1.48 to 526.12; participants = 40; trials = 1). None of the trials reported health-related quality of life, liver transplantation, decompensated liver disease, cirrhosis, or hepatocellular carcinoma. The proportion of people with chronic HCV infection as indicated by the lack of sustained virological response was lower in the interferon-alpha group versus no intervention (OR 0.27, 95% CI 0.09 to 0.76; participants = 99; trials = 3; I2 = 0%). The differences between the groups were imprecise or not estimable (because neither group had any events) for all the remaining comparisons.Four of the 10 trials (40%) received financial or other assistance from pharmaceutical companies who would benefit from the findings of the research; the source of funding was not available in five trials (50%), and one trial (10%) was funded by a hospital.
Authors' conclusions: Very low quality evidence suggests that interferon-alpha may decrease the incidence of chronic HCV infection as measured by sustained virological response. However, the clinical impact such as improvement in health-related quality of life, reduction in cirrhosis, decompensated liver disease, and liver transplantation has not been reported. It is also not clear whether this finding is applicable in the current clinical setting dominated by the use of pegylated interferons and direct-acting antivirals, although we found no evidence to support that pegylated interferons or ribavirin or both are effective in people with acute HCV infection. We could find no randomised trials comparing direct-acting antivirals with placebo or other interventions for acute HCV infection. There is significant uncertainty in the benefits and harms of the interventions, and high-quality randomised clinical trials are required.
Conflict of interest statement
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
MK: no financial disclosures. EB: no financial disclosures. DT: Astellas funded Douglas Thorburn for his attendance at the International Liver Transplantation Society meeting in 2014. Douglas Thorburn has also received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. There are no other financial disclosures to report. BD: no financial disclosures. ET: no financial disclosures. KG: no financial disclosures.
Figures







References
References to studies included in this review
-
- Calleri G, Colombatto P, Gozzelino M, Chieppa F, Romano P, Delmastro B, et al. Natural beta interferon in acute type‐C hepatitis patients: a randomized controlled trial. Italian Journal of Gastroenterology and Hepatology 1998;30(2):181‐4. - PubMed
-
- Csatary LK, Telegdy L, Gergely P, Bodey B, Bakacs T. Preliminary report of a controlled trial of MTH‐68/B virus vaccine treatment in acute B and C hepatitis: a phase II study. Anticancer Research 1998;18(2B):1279‐82. - PubMed
-
- Deterding K, Gruner N, Buggisch P, Wiegand J, Gale PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non‐inferiority trial. Lancet Infectious Diseases 2013;13(6):497‐506. - PubMed
-
- Genesca J. Interferon alfa in acute posttransfusion hepatitis C: a randomized, controlled trial. Gastroenterology 1992;103(5):1702‐3. - PubMed
- Genesca J, Esteban JI, Quer J, Viladomiu LL, Gonzalez A, Esteban R, et al. Hepatitis C virus markers in patients with acute post‐transfusion hepatitis treated with interferon alfa‐2b. Gut 1993;34(2 Suppl):S62‐3. - PMC - PubMed
-
- Hwang SJ, Lee SD, Chan CY, Lu RH, Lo KJ. A randomized controlled trial of recombinant interferon alpha‐2b in the treatment of Chinese patients with acute post‐transfusion hepatitis C. Journal of Hepatology 1994;21(5):831‐6. - PubMed
- Hwang SJ, Lee SD, Lee YH, Wu JC, Chan CY, Huang YS, et al. A randomized controlled clinical trial of recombinant interferon alpha‐2b in the treatment of acute post‐transfusion hepatitis C: a preliminary report. Journal of Gastroenterology and Hepatology 1993;8:S92‐8.
Additional references
-
- American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C, 2014. www.hcvguidelines.org/fullreport (accessed 12 October 2014). - PMC - PubMed
-
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, et al. Rational design of a potent, long‐lasting form of interferon: a 40 kDa branched polyethylene glycol‐conjugated interferon alpha‐2a for the treatment of hepatitis C. Bioconjugate Chemistry 2001;12(2):195‐202. - PubMed
-
- Beinhardt S, Aberle JH, Strasser M, Dulic‐Lakovic E, Maieron A, Kreil A, et al. Serum level of IP‐10 increases predictive value of IL28B polymorphisms for spontaneous clearance of acute HCV infection. Gastroenterology 2012;142(1):78‐85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

