Integrative Proteomics and Phosphoproteomics Profiling Reveals Dynamic Signaling Networks and Bioenergetics Pathways Underlying T Cell Activation
- PMID: 28285833
- PMCID: PMC5466820
- DOI: 10.1016/j.immuni.2017.02.010
Integrative Proteomics and Phosphoproteomics Profiling Reveals Dynamic Signaling Networks and Bioenergetics Pathways Underlying T Cell Activation
Abstract
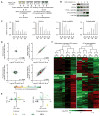
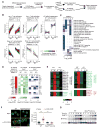
The molecular circuits by which antigens activate quiescent T cells remain poorly understood. We combined temporal profiling of the whole proteome and phosphoproteome via multiplexed isobaric labeling proteomics technology, computational pipelines for integrating multi-omics datasets, and functional perturbation to systemically reconstruct regulatory networks underlying T cell activation. T cell receptors activated the T cell proteome and phosphoproteome with discrete kinetics, marked by early dynamics of phosphorylation and delayed ribosome biogenesis and mitochondrial activation. Systems biology analyses identified multiple functional modules, active kinases, transcription factors and connectivity between them, and mitochondrial pathways including mitoribosomes and complex IV. Genetic perturbation revealed physiological roles for mitochondrial enzyme COX10-mediated oxidative phosphorylation in T cell quiescence exit. Our multi-layer proteomics profiling, integrative network analysis, and functional studies define landscapes of the T cell proteome and phosphoproteome and reveal signaling and bioenergetics pathways that mediate lymphocyte exit from quiescence.
Keywords: T cell; glycolysis; kinase; mTORC1; mitochondria; phosphoproteomics; proteomics; quiescence exit; systems biology; systems immunology.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

