Targeting BMI1+ Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma
- PMID: 28285905
- PMCID: PMC5419860
- DOI: 10.1016/j.stem.2017.02.003
Targeting BMI1+ Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma
Abstract
Squamous cell carcinoma in the head and neck (HNSCC) is a common yet poorly understood cancer, with adverse clinical outcomes due to treatment resistance, recurrence, and metastasis. Putative cancer stem cells (CSCs) have been identified in HNSCC, and BMI1 expression has been linked to these phenotypes, but optimal treatment strategies to overcome chemotherapeutic resistance and eliminate metastases have not yet been identified. Here we show through lineage tracing and genetic ablation that BMI1+ CSCs mediate invasive growth and cervical lymph node metastasis in a mouse model of HNSCC. This model and primary human HNSCC samples contain highly tumorigenic, invasive, and cisplatin-resistant BMI1+ CSCs, which exhibit increased AP-1 activity that drives invasive growth and metastasis of HNSCC. Inhibiting AP-1 or BMI1 sensitized tumors to cisplatin-based chemotherapy, and it eliminated lymph node metastases by targeting CSCs and the tumor bulk, suggesting potential regimens to overcome resistance to treatments and eradicate HNSCC metastasis.
Keywords: AP-1; Bmi1; cancer stem cells; chemotherapy; cisplatin resistance; head and neck squamous cell carcinoma; metastasis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
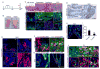



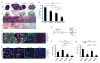


Comment in
-
Joining Forces: Bmi1 Inhibition and Cisplatin Curb Squamous Carcinogenesis.Cell Stem Cell. 2017 May 4;20(5):575-577. doi: 10.1016/j.stem.2017.04.008. Cell Stem Cell. 2017. PMID: 28475877
Similar articles
-
Co-targeting BMI1 and MYC to eliminate cancer stem cells in squamous cell carcinoma.Cell Rep Med. 2025 May 20;6(5):102077. doi: 10.1016/j.xcrm.2025.102077. Epub 2025 Apr 15. Cell Rep Med. 2025. PMID: 40239645 Free PMC article.
-
MicroRNA-200c attenuates tumour growth and metastasis of presumptive head and neck squamous cell carcinoma stem cells.J Pathol. 2011 Mar;223(4):482-95. doi: 10.1002/path.2826. Epub 2011 Jan 5. J Pathol. 2011. PMID: 21294122
-
Generation of a squamous cell carcinoma mouse model for lineage tracing of BMI1+ cancer stem cells.STAR Protoc. 2021 Apr 18;2(2):100484. doi: 10.1016/j.xpro.2021.100484. eCollection 2021 Jun 18. STAR Protoc. 2021. PMID: 33982017 Free PMC article.
-
Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences.Stem Cells. 2012 Mar;30(3):372-8. doi: 10.1002/stem.1035. Stem Cells. 2012. PMID: 22252887 Review.
-
C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell.Oral Oncol. 2014 Jul;50(7):633-9. doi: 10.1016/j.oraloncology.2014.04.004. Epub 2014 May 15. Oral Oncol. 2014. PMID: 24835851 Review.
Cited by
-
BMI1 Inhibition Eliminates Residual Cancer Stem Cells after PD1 Blockade and Activates Antitumor Immunity to Prevent Metastasis and Relapse.Cell Stem Cell. 2020 Aug 6;27(2):238-253.e6. doi: 10.1016/j.stem.2020.06.022. Epub 2020 Jul 21. Cell Stem Cell. 2020. PMID: 32697949 Free PMC article.
-
Comprehensive Exome Analysis of Immunocompetent Metastatic Head and Neck Cancer Models Reveals Patient Relevant Landscapes.Cancers (Basel). 2020 Oct 12;12(10):2935. doi: 10.3390/cancers12102935. Cancers (Basel). 2020. PMID: 33053752 Free PMC article.
-
SPP1 Derived from Macrophages Is Associated with a Worse Clinical Course and Chemo-Resistance in Lung Adenocarcinoma.Cancers (Basel). 2022 Sep 8;14(18):4374. doi: 10.3390/cancers14184374. Cancers (Basel). 2022. PMID: 36139536 Free PMC article.
-
PP2A and cancer epigenetics: a therapeutic opportunity waiting to happen.NAR Cancer. 2022 Feb 1;4(1):zcac002. doi: 10.1093/narcan/zcac002. eCollection 2022 Mar. NAR Cancer. 2022. PMID: 35118387 Free PMC article.
-
Targeting Cancer Stem Cells to Overcome Chemoresistance.Int J Mol Sci. 2018 Dec 13;19(12):4036. doi: 10.3390/ijms19124036. Int J Mol Sci. 2018. PMID: 30551640 Free PMC article. Review.
References
-
- Belguise K, Kersual N, Galtier F, Chalbos D. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene. 2005;24:1434–1444. - PubMed
-
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. - PubMed
-
- Chakrabarti R, Wei Y, Hwang J, Hang X, Andres Blanco M, Choudhury A, Tiede B, Romano RA, DeCoste C, Mercatali L, et al. DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol. 2014;16:1004–1015. 1001–1013. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

