Wnt/TLR Dialog in Neuroinflammation, Relevance in Alzheimer's Disease
- PMID: 28286503
- PMCID: PMC5323396
- DOI: 10.3389/fimmu.2017.00187
Wnt/TLR Dialog in Neuroinflammation, Relevance in Alzheimer's Disease
Abstract
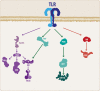
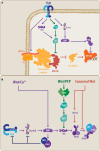
The innate immune system (IIS) represents the first line of defense against exogenous and endogenous harmful stimuli. Different types of pathogens and diverse molecules can activate the IIS via a ligand-receptor mechanism. Cytokine release, recruitment of immunocompetent cells, and inflammation constitute the initial steps in an IIS-mediated response. While balanced IIS activity can resolve a harmful event, an altered response, such as deficient or persistent IIS activity, will have a critical effect on organism homeostasis. In this regard, chronic IIS activation has been associated with a wide range of diseases, including chronic inflammatory disorders (inflammatory bowel disease, arthritis, chronic obstructive pulmonary disease, among others), cancer and, more recently, neurodegenerative disorders. The relevance of the immune response, particularly inflammation, in the context of neurodegeneration has motivated rigorous research focused on unveiling the mechanisms underlying this response. Knowledge regarding the molecular hallmarks of the innate immune response and understanding signaling pathway cross talk are critical for developing new therapeutic strategies aimed at modulating the neuroinflammatory response within the brain. In the present review, we discuss the IIS in the central nervous system, particularly the cross talk between the toll-like receptor-signaling cascade and the wingless-related MMTV integration site (Wnt) signaling pathway and its relevance in neurodegenerative disorders such as Alzheimer's disease.
Keywords: Alzheimer’s disease; Wnt signaling; immune response; neuroinflammation; toll-like receptors.
Figures
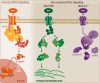
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

