Spina Bifida: Pathogenesis, Mechanisms, and Genes in Mice and Humans
- PMID: 28286691
- PMCID: PMC5327787
- DOI: 10.1155/2017/5364827
Spina Bifida: Pathogenesis, Mechanisms, and Genes in Mice and Humans
Abstract
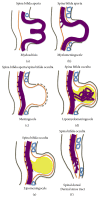
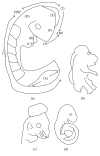
Spina bifida is among the phenotypes of the larger condition known as neural tube defects (NTDs). It is the most common central nervous system malformation compatible with life and the second leading cause of birth defects after congenital heart defects. In this review paper, we define spina bifida and discuss the phenotypes seen in humans as described by both surgeons and embryologists in order to compare and ultimately contrast it to the leading animal model, the mouse. Our understanding of spina bifida is currently limited to the observations we make in mouse models, which reflect complete or targeted knockouts of genes, which perturb the whole gene(s) without taking into account the issue of haploinsufficiency, which is most prominent in the human spina bifida condition. We thus conclude that the need to study spina bifida in all its forms, both aperta and occulta, is more indicative of the spina bifida in surviving humans and that the measure of deterioration arising from caudal neural tube defects, more commonly known as spina bifida, must be determined by the level of the lesion both in mouse and in man.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Harris M. J., Juriloff D. M. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(8):653–669. doi: 10.1002/bdra.20676. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

